Figures & data
Table 1 Characteristics of Seventeen Population-Based Studies Included for the Comparison of the Sex- and Age-Specific Incidence of Myocarditis and/or Pericarditis Following mRNA COVID-19 Vaccinations
Table 2 Number of Cases, Proportion of Myocarditis with or without Pericarditis, Definite or Probable Cases, Cases Occurring 0–7 Days After Vaccination Over Total Cases Among Seventeen Population-Based Studies
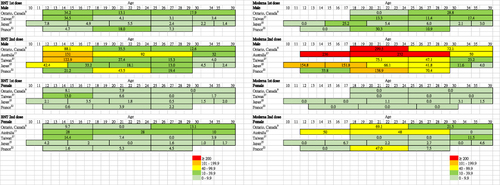
Figure 1 Reported rates of myocarditis (per million persons) at 0–7 days after the first and second doses of BNT162b2 (BNT) and mRNA-1273 (Moderna) vaccine according to sex and age group.
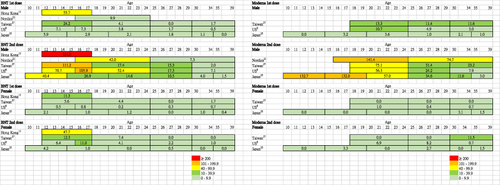
Figure 2 Reported rates of myocarditis (per million persons) at 0–21 or 28 days after the first and second doses of BNT162b2 (BNT) and mRNA-1273 (Moderna) vaccine according to sex and age group.

Figure 3 Reported rates of myocarditis (per million persons) without restriction on time after the first and second doses of BNT162b2 (BNT) and mRNA-1273 (Moderna) vaccine according to sex and age group.