Figures & data
Table 1 Definitions of self-care acknowledged in literature
Table 2 Type of nonprescription medicines in UK and Japan
Figure 1 Approval processes in the UK and Japan.
Abbreviations: MHLW, Ministry of Health, Labour and Welfare; OTC, over-the-counter; MHRA, Medicines and Healthcare products Regulatory Agency; PMDA, pharmaceuticals and medical devices agency.
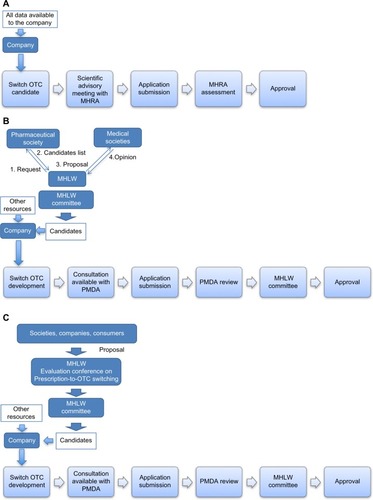
Table 3 The list of substances reclassified from nonprescription medicines to pharmacy or general sale medicines commonly in the UK and Japan, from 1983 to January 2015
