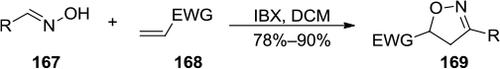Figures & data
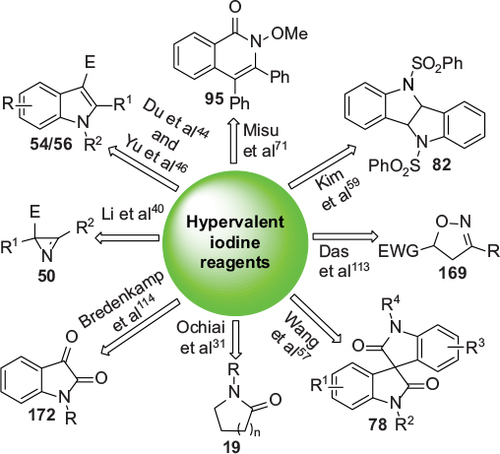
Figure 2 Representative hypervalent iodine (III) reagents.
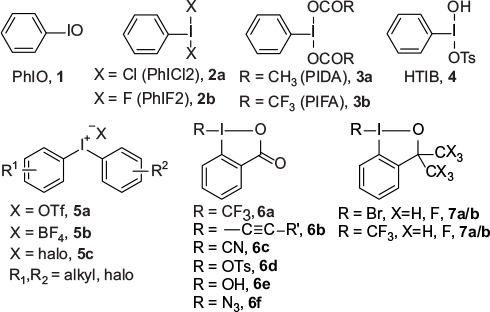
Figure 3 (A) PhIO-mediated construction of thiaozles, imidazoles, and imidazo[1,2-a]pyridines. (B) Proposed mechanism of the oxidation reaction in step I.
![Figure 3 (A) PhIO-mediated construction of thiaozles, imidazoles, and imidazo[1,2-a]pyridines. (B) Proposed mechanism of the oxidation reaction in step I.](/cms/asset/f4afaaf8-5f6c-4a79-a546-99aefc916e3f/droc_a_84894_f0003_b.jpg)
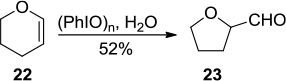
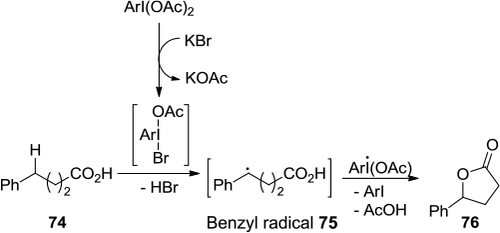
Figure 4 (A) (a) PhIO-mediated synthesis of three-membered ring 12 and five-membered ring 13. (b) PhIO-mediated synthesis of oxetane 15. (c) PhIO-mediated synthesis of azetidine 17 (B) Proposed mechanism of (a) and (c).
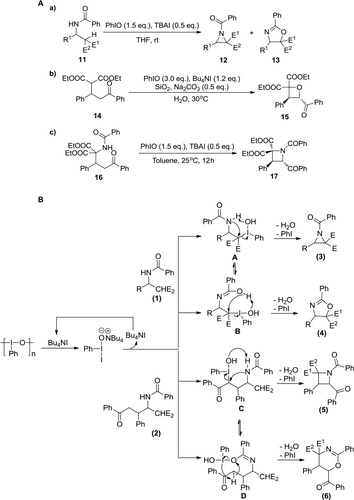
Figure 5 PhIO-mediated functionalization of cyclic amines.
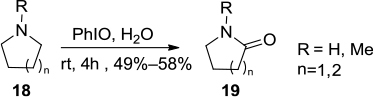
Figure 6 PhIO-mediated oxidation affording α-methoxylated carbonyl compounds.
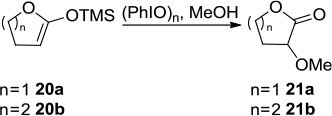
Figure 8 Synthesis of the homochiral 7-oxa-2-azabicyclo[2.2.1]heptane ring system.
![Figure 8 Synthesis of the homochiral 7-oxa-2-azabicyclo[2.2.1]heptane ring system.](/cms/asset/222f97b8-09c3-47dd-b987-16458c4c92dc/droc_a_84894_f0008_b.jpg)
Figure 9 PhIO-mediated conversion of proline into 2-pyrrolidone in nonpolar solvent.
Figure 10 Synthesis of various 1-fluoroglycosides with TolIF2.
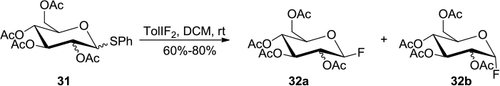
Figure 11 Ring-expansion reactions induced by TolIF2.
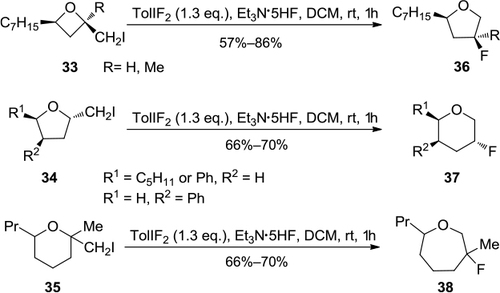
Figure 12 Synthesis of α-hydroxy-β,β-dichloropyrrolidine with 4-NO2PhICl2.
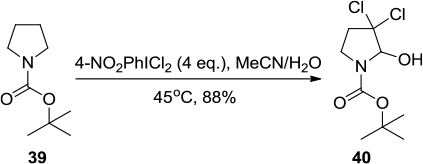
Figure 13 PhICl2/Pb(SCN)2-mediated thiocyanation of enol silyl ethers leading to lactone 42.
Figure 14 Lewis base-catalyzed chlorination facilitated by PhICl2.
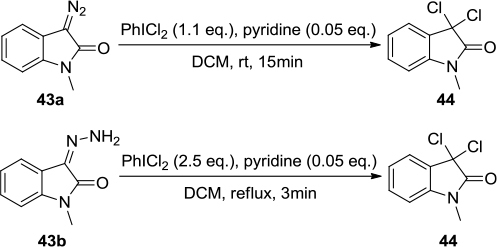
Figure 15 (A) Direct synthesis of oxazolidin-2-ones and imidazolidin-2-ones using PhICl2 and NaN3. (B) Proposed mechanism.
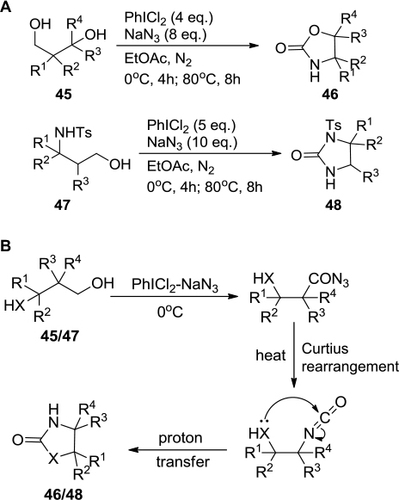
Figure 16 PIDA-mediated synthesis of 2H-azirine derivatives from enamines.
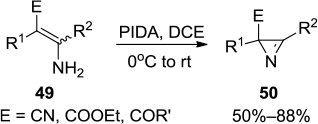
Figure 17 PIFA-mediated synthesis of polysubstituted pyrroles 52.
Figure 18 (A) I (III)-mediated synthesis of indoles from enamines 53. (B) I (III)-mediated synthesis of indoles from enamines 55.
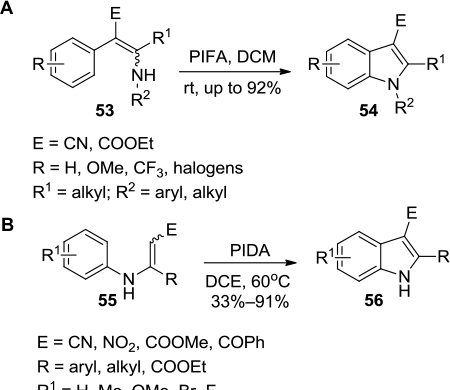
Figure 19 (A) PIDA-mediated synthesis of imidazoles via condensation of α-hydroxy ketones with aldehydes and NH4OAc. (B) Proposed mechanism.
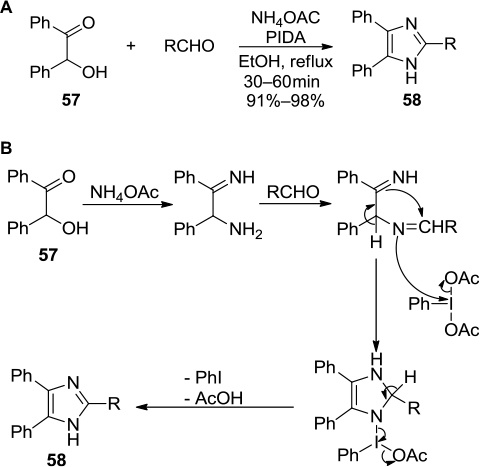
Figure 20 PIDA-mediated synthesis of aminoindazole derivatives.
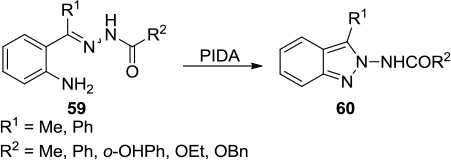
Figure 21 PIFA/TMSOTf-mediated synthesis of benzoxazole derivatives.

Figure 22 (A) PIFA-mediated synthesis of 2-trifluoromethyl oxazole derivatives. (B) Proposed mechanism.
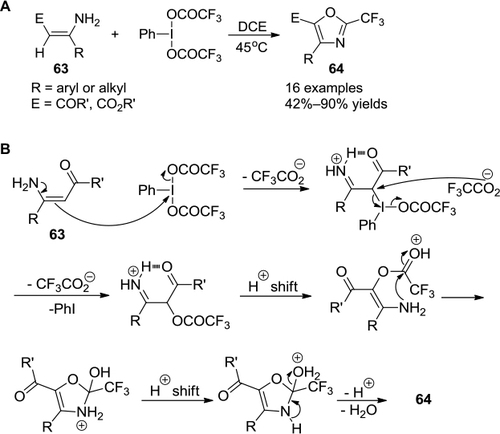
Figure 23 PIDA-mediated synthesis of 2,5-disubstituted oxazoles in AcOH or AcOH-HFIP.
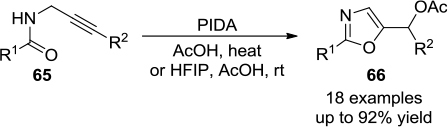
Figure 24 PIDA/KOH-mediated synthesis of 2-benzimidazolones and 2-benzoxazolones.
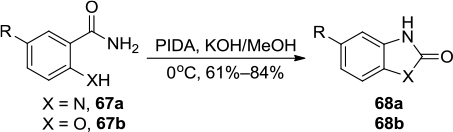
Figure 25 (A) PIFA-mediated intramolecular synthesis of benzothiazoles. (B) PIDA-mediated intermolecular synthesis of benzothiazoles.
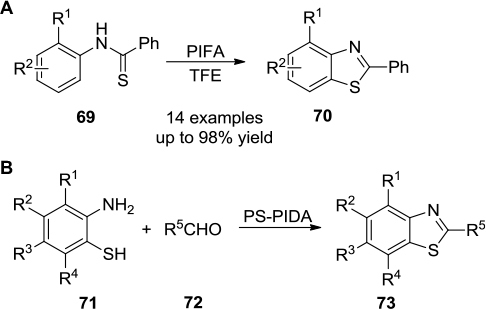
Figure 27 (A) Metal-free synthesis of spirooxindoles via PIFA-mediated cascade oxidation. (B) Proposed mechanism.
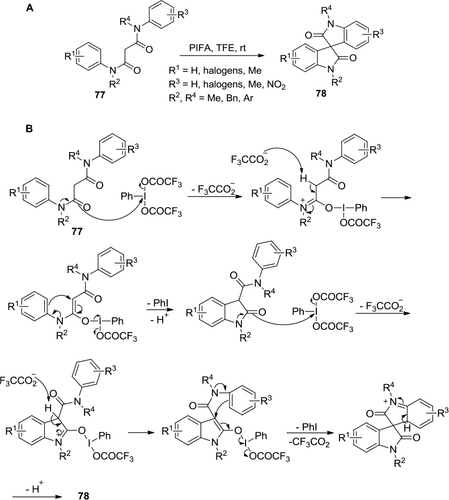
Figure 28 (A) PIFA-mediated conversion of internal alkynes to spiro heterocycles via cascade annulation. (B) Proposed mechanism.
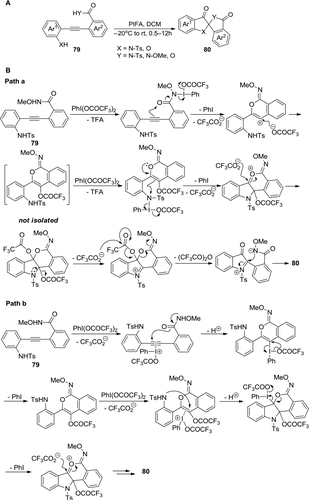
Figure 29 (A) PIDA-mediated synthesis of bisindolines via cascade intramolecular oxidative deamination. (B) Proposed mechanism.
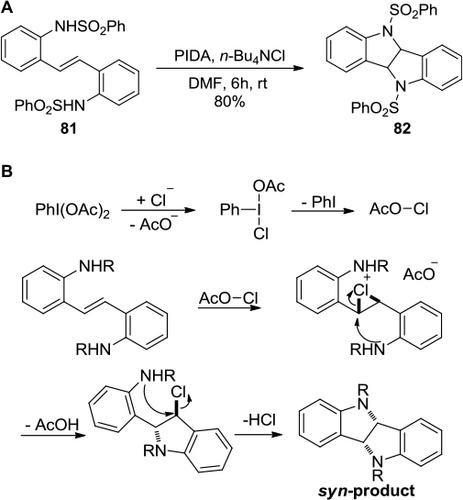
Figure 30 PIFA-mediated synthesis of benzo[c]phenanthridine and phenanthridinone.
![Figure 30 PIFA-mediated synthesis of benzo[c]phenanthridine and phenanthridinone.](/cms/asset/ab731a66-e864-4eb0-8706-ee8c54d33ec8/droc_a_84894_f0030_b.jpg)
Figure 31 PIFA-mediated synthesis of 3-arylquinolin-2-ones from N-methyl-N-phenylcinnamamides through oxidative C–C bond formation/1,2-aryl migration.
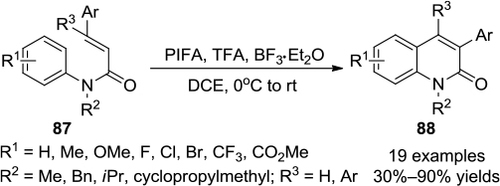
Figure 32 PIFA-mediated direct intramolecular cyclization of α-(aryl)alkyl-β-dicarbonyl compounds.
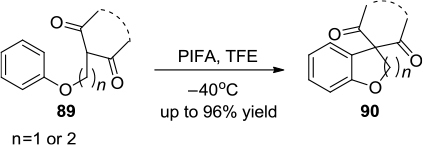
Figure 33 PIFA-mediated synthesis of N-aryl-N-methoxyamides via an intramolecular oxidative C–N bond formation.
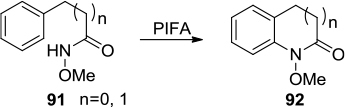
Figure 34 Synthesis of isoquinolones from N-methoxybenzamide and diphenyl acetylene mediated by PIDA generated in situ.
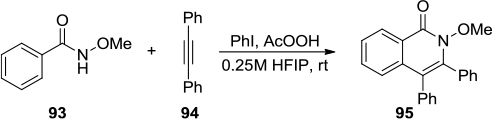
Figure 35 (A) PIFA-mediated synthesis of the fused indeno-1,4-diazepinones. (B) Proposed mechanism.
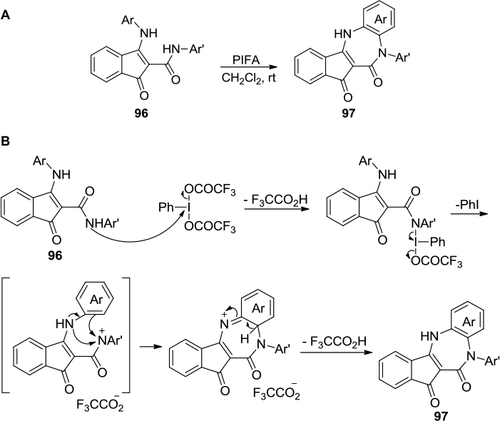
Figure 36 I (III)-mediated formation of dibenzodihydro-1,3-diazepin-2-ones and dibenzo[d,f][1,3]oxazepin-6(7H)-ones.
![Figure 36 I (III)-mediated formation of dibenzodihydro-1,3-diazepin-2-ones and dibenzo[d,f][1,3]oxazepin-6(7H)-ones.](/cms/asset/467a317b-27bc-4d3a-999f-775b1042633a/droc_a_84894_f0036_b.jpg)
Figure 37 PIFA/I2-mediated iodination of indole derivatives to 3-iodoindoles 103.
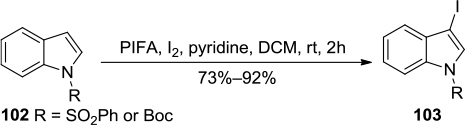
Figure 38 PIFA/TMSCN-mediated selective cyanation of N-tosylpyrroles at the C2 position.
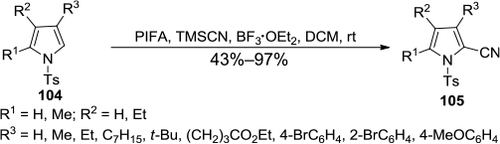
Figure 39 PIDA-mediated homogeneous azidization and selenylation of glycals.
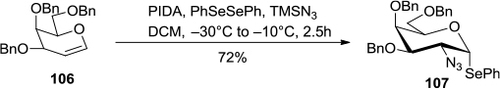
Figure 40 HTIB-mediated synthesis of trifluoromethyl-2-isoxazoline-N-oxides.
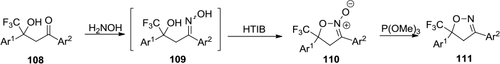
Figure 41 HTIB-mediated synthesis of 4-methoxy-2H-chromene.
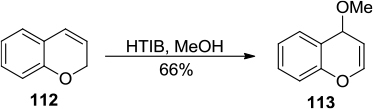
Figure 42 Intramolecular carbotrifluoromethylation of alkynes with Togni’s reagent and Cu(I).
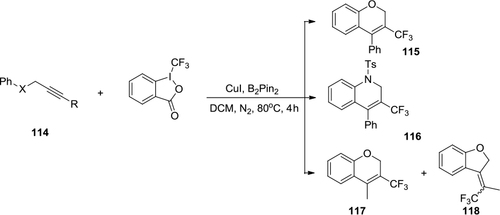
Figure 43 Trifluoromethylation of indole derivatives with Togni’s reagent.
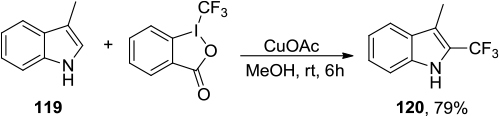
Figure 44 (A) Synthesis of biologically important 1-trifluoromethylated isoquinolines with Togni’s reagent. (B) Proposed mechanism.
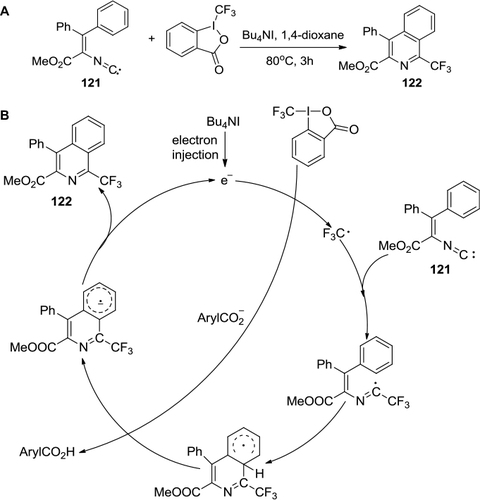
Figure 45 Aryltrifluoromethylation of N-phenylcinnamamides by using Togni’s reagent and copper catalyst.
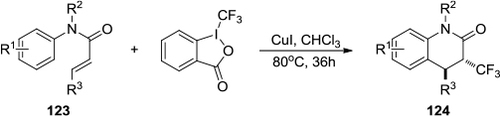
Figure 46 Direct alkynylation of indole and pyrrole heterocycles by using TIPS-EBX.
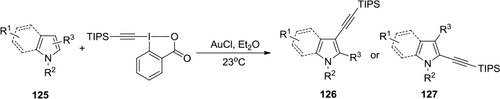
Figure 47 Selective cobalt(III)-catalyzed alkynylation of indoles using hypervalent iodine-alkyne reagents.
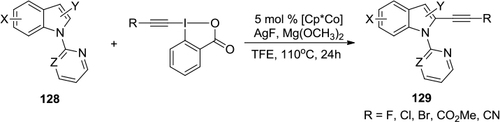
Figure 49 (A) Cycloaddition of ortho-silyl aryltriflates and iodonium ylides. (B) Proposed mechanism.
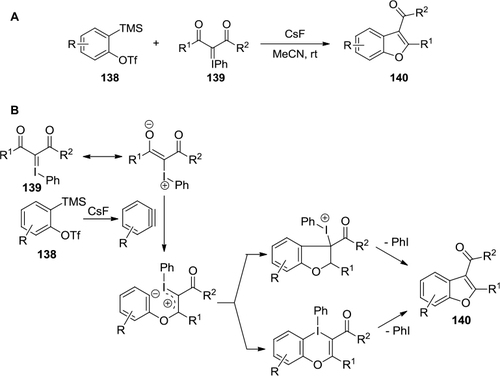
Figure 51 Diaryliodonium salts-mediated arylation of indoles at C2.
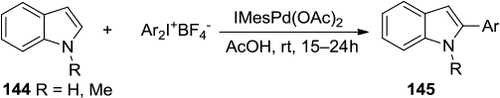
Figure 52 Cu-catalyzed tandem C–H/N–H arylation of indoles with diaryliodonium salts.
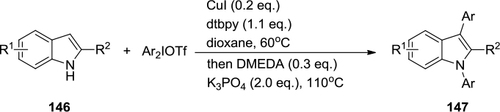
Figure 53 Arylation of N-containing heterocycles with diaryliodonium salts.
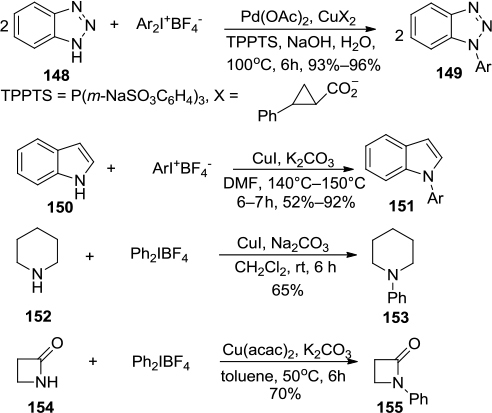
Figure 54 A Cu(OTf)2-catalyzed, three-component regioselective synthesis of polysubstituted quinolones.
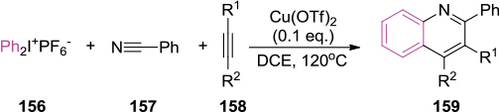
Figure 55 Oxidative cleavage of the glycol C–C bond with DMP.
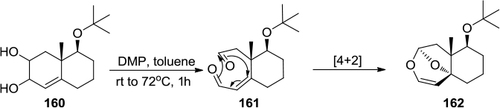
Figure 56 Synthesis of 2-substituted benzothiazoles with DMP.
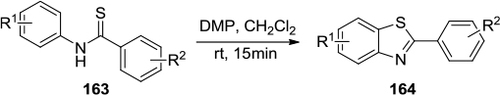
Figure 57 IBX-mediated stereoselective 5-exo and 6-exo formations of isoxazolidines and [1,2]oxazinanes.
![Figure 57 IBX-mediated stereoselective 5-exo and 6-exo formations of isoxazolidines and [1,2]oxazinanes.](/cms/asset/6ccd715b-8d92-4c12-8d34-0454b37de467/droc_a_84894_f0057_b.jpg)