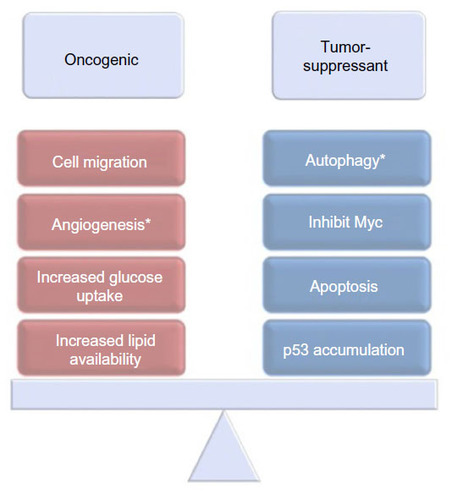
Figures & data
Table 1 A representative list of proteins whose expression is regulated by HIF-1
Table 2 Positive and negative regulators of NO production
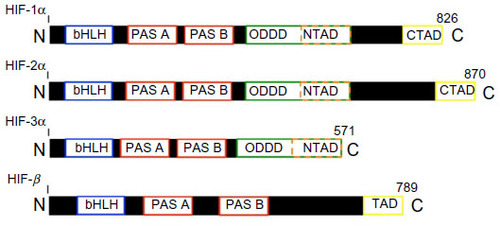
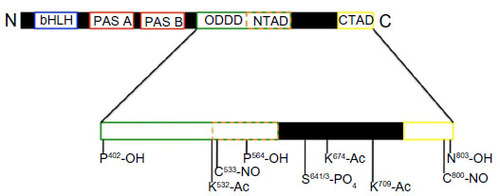
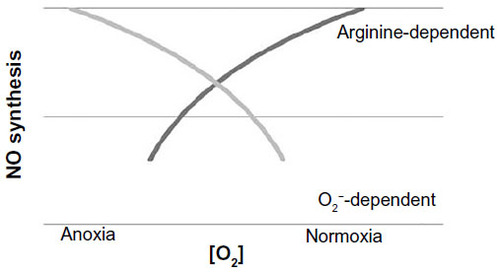
-dependent NO synthesis.