Figures & data
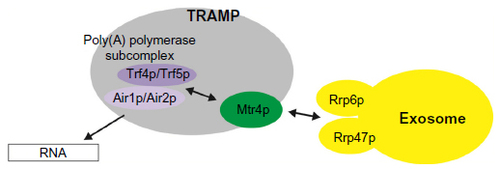
Figure 1 Model of interactions between RNA, TRAMP, and the exosome. RNA substrate bound by Air1p or Air2p is oligo-adenylated by poly(A) polymerase Trf4p or Trf5p until sufficient binding sites are generated for the helicase Mtr4p. Mtr4p is loosely associated with the poly(A) polymerase subcomplex in the TRAMP complex. Mtr4p can only fully bind when the 3′ extensions contain at least 5–6 nucleotides. Therefore, during polyadenylation, the binding affinity of Mtr4p to target substrate increases. This increased affinity might contribute to Mtr4p inhibiting further oligo-adenylation and might induce slight slowing of TRAMP dissociation from the substrate. TRAMP recruits the exosome for RNA degradation through the interaction between Mtr4p and Rrp6p/Rrp47p. Mtr4p also forms a complex with other proteins, such as with Hrb1p and Gbp2p, to mediate quality control of spliced transcripts in yeast, and with hRbm7p and hZCCHC8, as NEXT complexes to degrade PROMPTs.