Figures & data
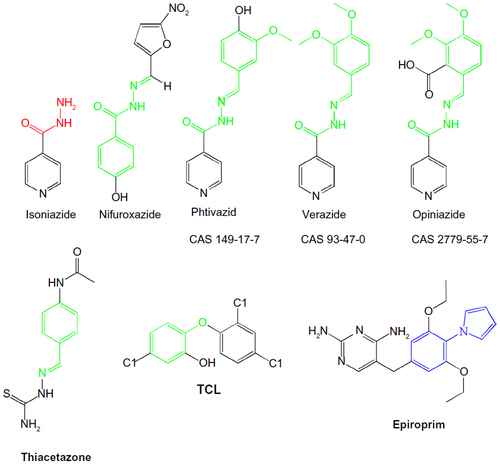
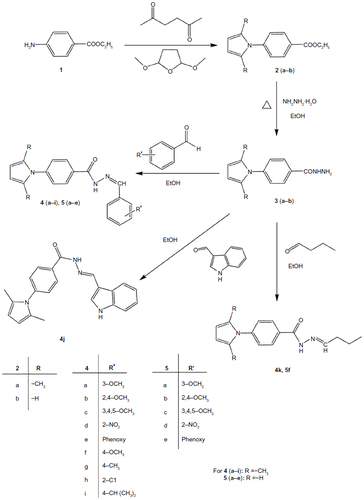
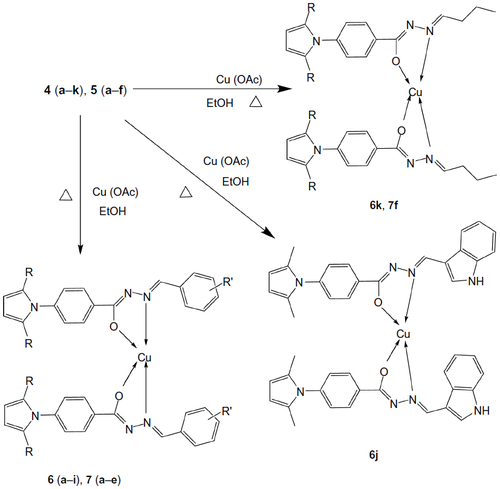
Figure 2 Designed anti-TB drugs (pyrrolyl Schiff bases and proposed structures of their complexes).
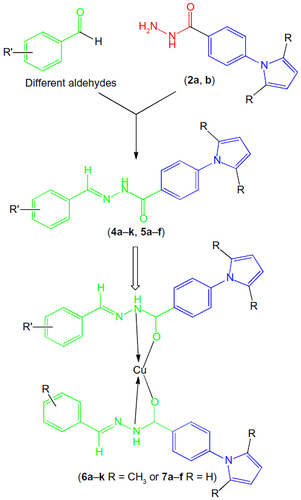
Figure 5 (A) and (B) Docked view of ligand (compound 4e) in B chain of PDB 2X22.
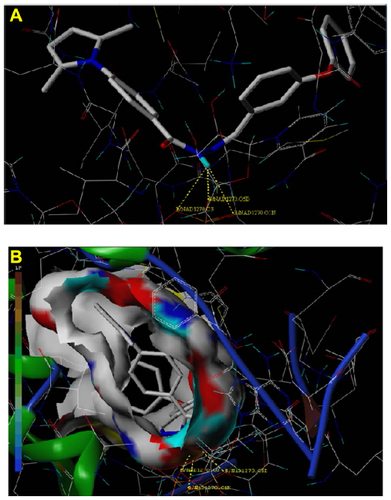
Figure 6 Docked view of ligand (compound 7d) in B chain of PDB 2X22.
Table 1 Surflex-Dock scores (kcal/mol) of N′-(arylidene)-4-(2,5-dimethyl-1H-pyrrol-1-yl)benzohydrazides, N′-(arylidene)-4-(1H-pyrrol-1-yl)benzohydrazides, and their copper complexes
Table 2 Antitubercular activities of synthesized compounds (4a–k, 5a–f, 6a–k, and 7a–f)
Table 3 In vitro antibacterial activity (MIC expressed in μg/mL)