Figures & data
Table 1 Secondary symptoms and complications of unmanaged constipation
Table 2 Gastrointestinal adverse events (%) after 4 weeks of treatment with the sublingual tablet formulations of buprenorphine–naloxone (16/4 mg) or buprenorphine (16 mg)
Figure 1 Constipation* at baseline and at week 12 in patients converted from SLBN to BBN (N=186).
Abbreviations: BBN, buccal buprenorphine-naloxone film; ET, early termination; SLBN, sublingual buprenorphine-naloxone tablets or films.
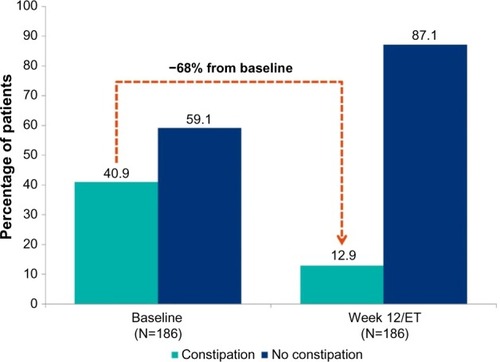
Table 3 Systemic exposure of 4.2/0.7 mg BBN film and 8/2 mg SLBN tablet (N=80)
Table 4 Predicted buprenorphine and norbuprenorphine exposure with SLBN tablets and BBN films: daily dosing and steady-state conditions
