Figures & data
Figure 1 Isolation process and potential therapeutic products derived from lipoaspirate.
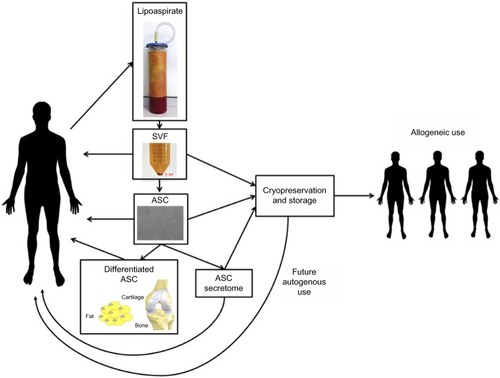
Figure 2 Fluorescence-activated cell sorting characterization of (A) nonhematopoietic (CD45-) cells of stromal vascular fraction (SVF) and (B) adherent purified adipose-derived stem cells (ASC). (C) Summary of flow cytometry cell surface marker expression analysis for uncultured endothelial progenitor cells (EPC) in SVF, uncultured ASC in SVF, and adherent purified ASC.
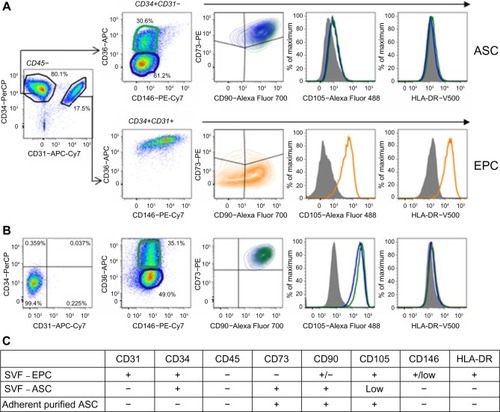
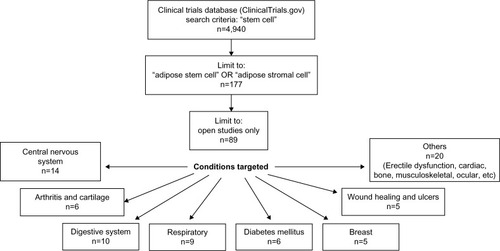
Figure 4 Clinical trials registered on http://www.ClinicalTrials.gov focused on adipose stem or stromal cells as at April 2015.