Figures & data
Table 1 List of Primers with Their Tm, GC Content, and Product Size Used for HBB Gene and Prevalent Mutations
Table 2 Genotype of 77 Clinically Manifested β-Thalassemia Major Patients (Homozygous and Compound Heterozygous)
Table 3 Genotypic Frequencies of Common and Rare Mutations Found in the Population of Western Region of Uttar Pradesh, India
Figure 1 Chromatograms of DNA sequences depicting the HBB Mutations at different positions. (A) Codon 3 (T>C) and (B) Codon 41/42 (-CTTT), mutations are present in exons, while (C–F) are the presentation of mutations that are present in intronic region of β-globin gene. Red arrows are used for marking the exact position of mutations. Sequence analysis was done using Finch TV 1.4.0. (FinchTV chromatogram viewer is a popular desktop application, developed by Geospiza, Inc.).
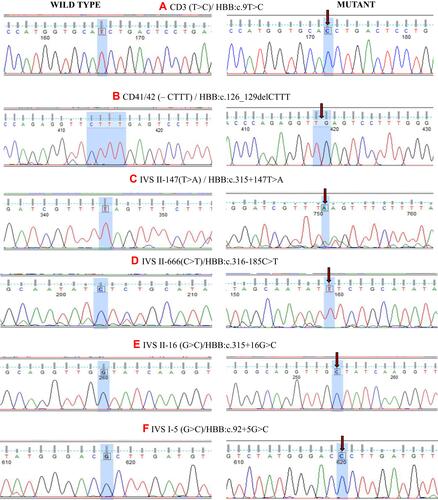
Figure 2 (A) Sanger sequencing chromatogram confirms the deletion of four nucleotides (AAAG) from beta-globin gene (HBB). (B) The peripheral blood smear stained with Leishman’s staining showing Anisopoikilocytosis; Acanthocytes (Irregularly spaced blunted projections from the margin of the cells) and Dacryocytes or Teardrop erythrocytes can be seen (marked by black arrows). (C) The Mview of Multiple Sequence Alignment (EMBL-EBI) of two beta-globin protein sequences; first one is of mutant with deletion of AAAG and second one is a normal protein sequence (control). This deletion is responsible for the frameshifting which results into stop codon and ultimately cause truncated β-globin chain (Rectangular region showing point of frameshifting). (D) The modelling using online software Swiss-Model shows the truncated protein of 87 aa has lost its binding capacity towards heme molecule as compared to normal beta-globin protein (147 aa). (i), (ii) and (iii) depicting model for normal beta-globin structure and its binding with heme molecule, whereas the figure (iv and v) shows truncated protein of 87 amino acids, which is devoid of all amino acids that are involved in binding with heme molecules.
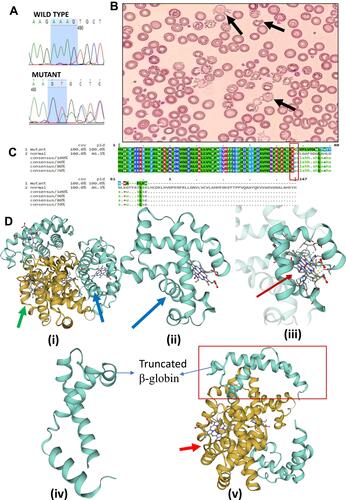
Table 4 Genotype-Phenotype Correlation: All the Patients Were Having Markedly Increased Level of S. Ferritin, HbA2, and HbF Level, and Decreased Values of MCV and MCH. Phenotypes of Patients with Silent Mutations Viz. Codon 3 (T>C)/IVS II-16 (G>C) or IVS II-147 (T>A) or IVS II-666 (C>T) Shows Possibilities of the Presence of Other Deleterious Mutations
