Figures & data
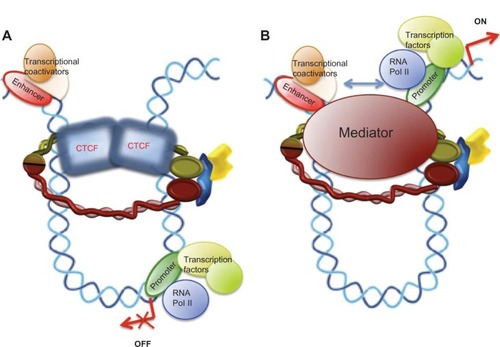
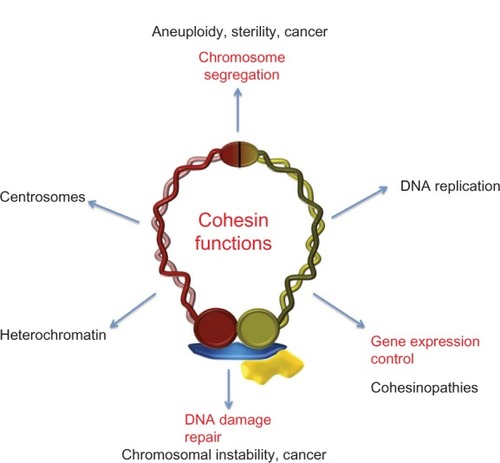
Figure 1 Cohesin complex and cohesin regulators.
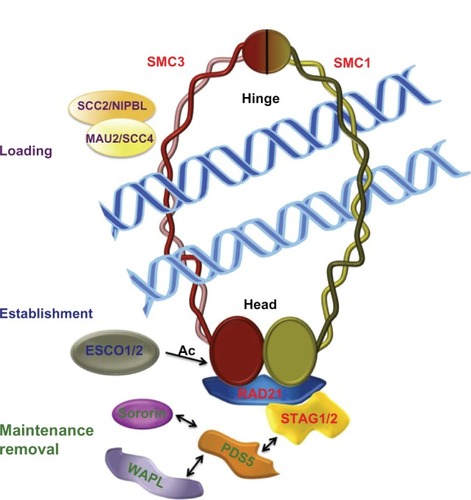
Table 1 Subunits of cohesin complexes and cohesin regulators implicated in cohesinopathies
Figure 2 Cohesin and cohesin regulators in human cohesinopathies.
Abbreviation: Ac, Acetylation.
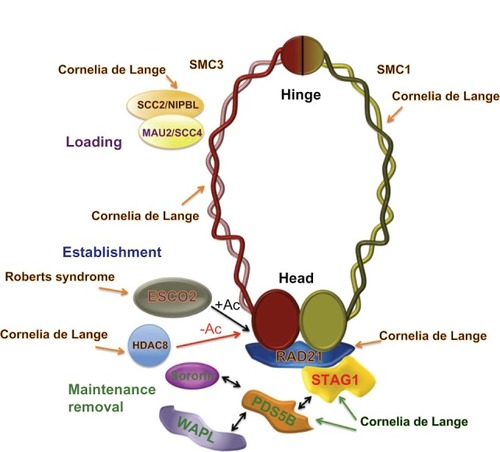
Figure 3 Models for a chromatin architectural function of cohesin in control of gene expression. (A) Interaction of the cohesin complex with chromatin-bound CTCF maintains a chromatin structure in which the enhancer cannot interact with the promoter and behaves as an insulator, repressing gene expression. (B) Loop structure formed by the mediator/cohesin complex allows enhancer-promoter interactions promoting gene transcription.