Figures & data
Figure 1 Concept of synthetic lethality explained with PARP inhibitors in the setting of BRCA mutation.
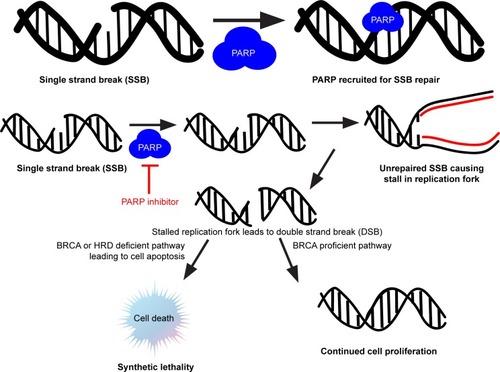
Figure 2 Study 10 design.
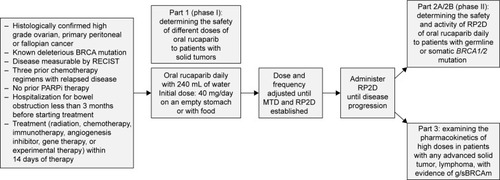
Figure 3 ARIEL2 study design.
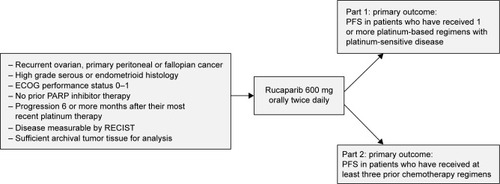
Figure 4 ARIEL3 study design.
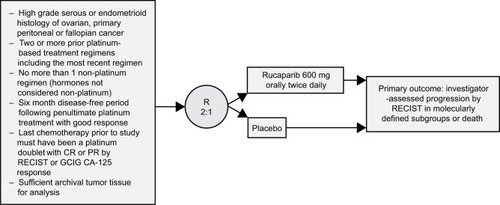
Figure 5 ARIEL3 efficacy analysis nested cohorts.
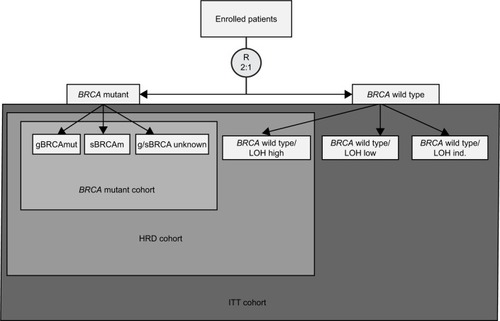
Table 1 Current rucaparib trials in ovarian cancer and other disease types
Table 2 Examples of other PARP inhibitors under phase III investigation in the US according to www.ClinicalTrials.gov
