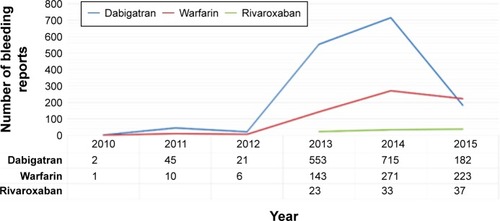
Figures & data
Table 1 Safety and efficacy endpoints for landmark clinical trials for studied drugs
Table 2 Demographic distribution of reports
Table 3 The ROR and associated 95% CIs for bleeding following dabigatran, rivaroxaban or warfarin use
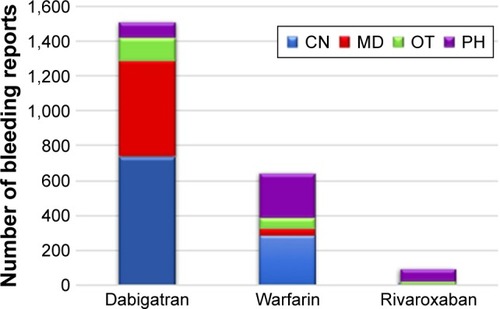
Figure 2 Number of bleeding reports associated with each drug stratified by reporter.