Figures & data
Table 1 Summary of baseline variables
Figure 1 Percentage change in bone mineral density in the lumbar spine from baseline (±95% CI) over 12 months in patients who started receiving AIs with denosumab (“With denosumab”) and those who had received AI before the initiation of denosumab therapy (“Before denosumab”).
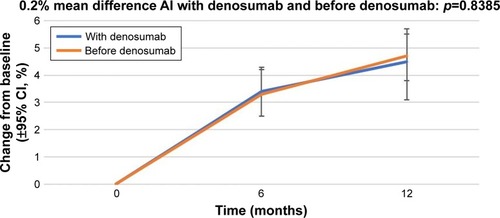
Figure 2 Percentage change in bone mineral density in the right femoral neck from baseline (±95% CI) over 12 months in patients who started receiving AIs with denosumab (“With denosumab”) and those who had received AI before the initiation of denosumab therapy (“Before denosumab”).
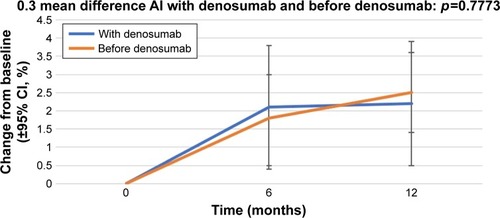
Figure 3 Percentage change in BMD in the left femoral neck from baseline (±95% CI) over 12 months in patients who started receiving AIs with denosumab (“With denosumab”) and those who had received AI before the initiation of denosumab therapy (“Before denosumab”).
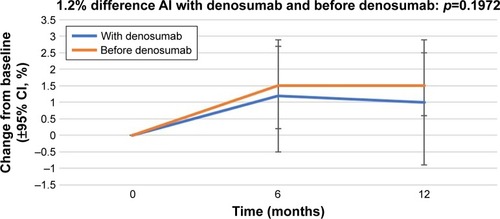
Table 2 Subgroup analyses of the therapeutic effects of denosumab at 12 months
