Figures & data
Table 1 Definitions of clinical response and microbiological response
Figure 1 Patient disposition.
Abbreviations: CE, clinically evaluable; cIAI, complicated intra-abdominal infection; c-mITT, clinical modified intent-to-treat; HIV, human immunodeficiency virus; ME, microbiologically evaluable; mITT, modified intent-to-treat; m-mITT, microbiological modified intent-to-treat; TOC, test-of-cure.
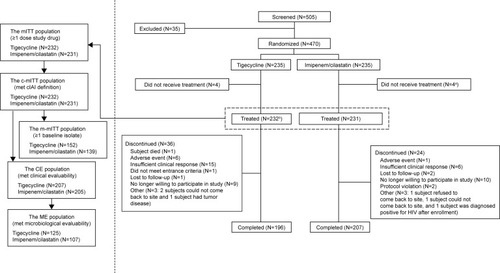
Table 2 Demographics and baseline characteristics of the CE population
Table 3 Clinical response at the test-of-cure assessment
Table 4 Microbiological response at the subject level in the test-of-cure assessment for the ME population
Table 5 Pathogen-level microbiological eradication rates by species or types of baseline isolates at the test-of-cure assessment for the ME population
Table 6 Summary of treatment-emergent adverse events in the safety analysis set
Table 7 Treatment-emergent adverse events in ≥2% of subjects in either treatment group in the safety analysis set
