Figures & data
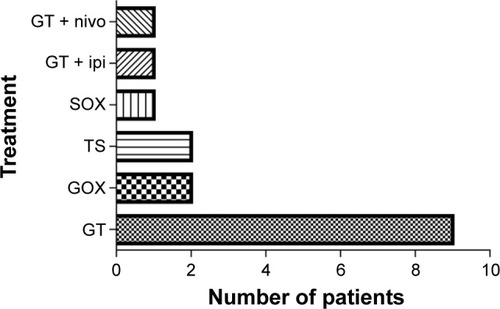
Table 1 Characteristics of patients with advanced pancreatic cancer received
Table 2 The efficacy of patients with advanced pancreatic cancer received PD-1/PD-L1 inhibitor
Figure 2 Tumor response.
Abbreviation: RECIST, Response Evaluation Criteria in Solid Tumors.
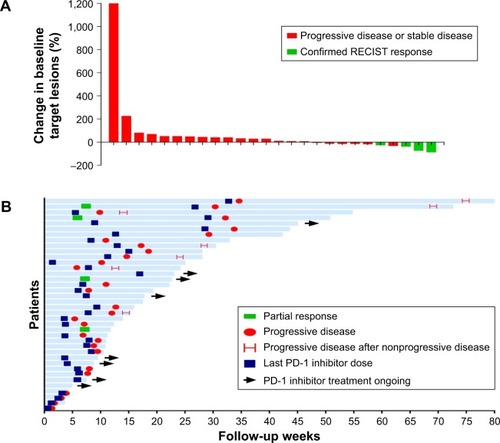
Figure 3 Kaplan–Meier estimates of progression-free survival and overall survival times.
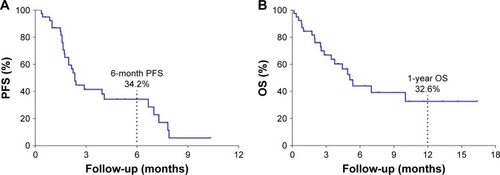
Table 3 The survival of patients with advanced pancreatic cancer received PD-1/PD-L1 inhibitor
Figure 4 Kaplan–Meier plots of progression-free survival and overall survival times in the full analytical set of patients with advanced pancreatic cancer who received the PD-1/PD-L1 inhibitor in different lines of treatment.
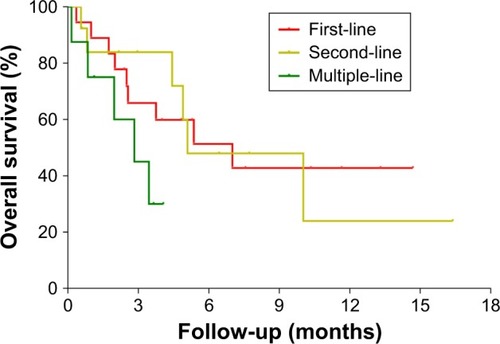
Table 4 Comparison of the survival of patients with advanced pancreatic cancer received PD-1/PD-L1 inhibitor in different lines of treatment
Figure 5 Kaplan–Meier estimates of progression-free survival and overall survival times.
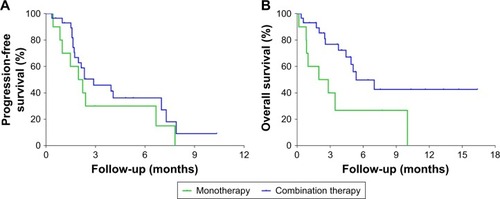
Table 5 Comparison of the efficacy and survival of patients with advanced pancreatic cancer received PD-1/PD-L1 inhibitor between monotherapy and combined therapy
Figure 6 Kaplan–Meier plots of progression-free survival in the full analytical set of patients with advanced pancreatic cancer who continued to receive the PD-1/PD-L1 inhibitor after the failure of PD-1/PD-L1 treatment.
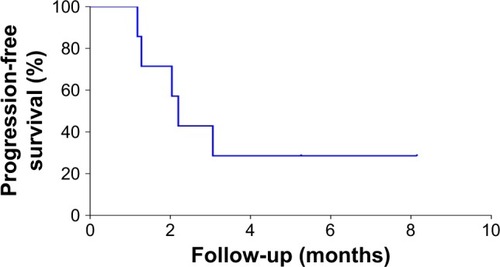
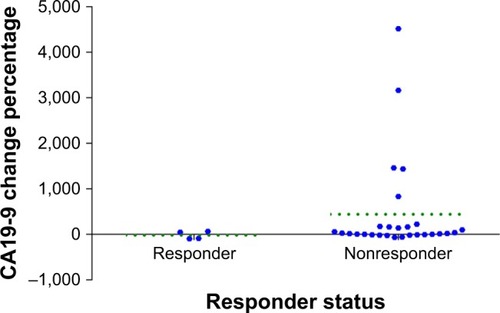
Table 6 The efficacy of patients with different CA19-9 level changes
Figure 8 The efficacy of treatment in patients with different levels of PD-L1 expression.
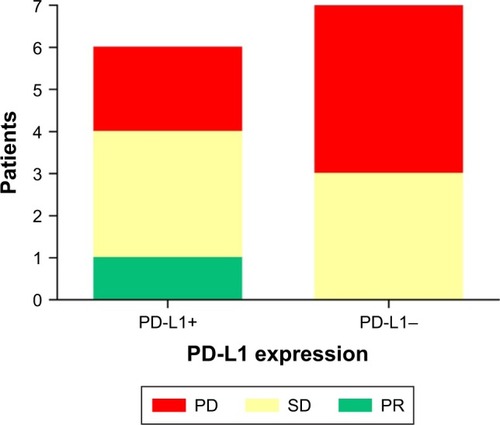
Table 7 The efficacy and survival of patients with genetic mutations
Table 8 Adverse events (AEs) in patients with advanced pancreatic cancer received immune checkpoint inhibitors