Figures & data
Table 1 Details about the drugs considered in this study
Figure 1 (A–D) Distribution of RR-MS population reporting adverse reactions treated with interferons, in relation to age and sex.
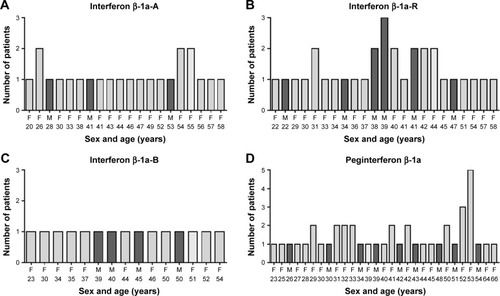
Figure 2 (A–E) Distribution of RR-MS population reporting adverse reactions in relation to age and sex.
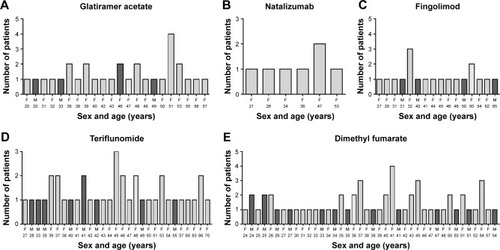
Figure 3 Total expected and unexpected adverse events for each drug evaluated.
Abbreviations: DMF, dimethyl fumarate; FTY720, fingolimod; GA, glatiramer acetate; IFN β-1a-A, interferon β-1a Avonex®; IFN β-1a-R, interferon β-1a Rebif®; IFN β-1b-B, interferon β-1b Betaferon®; IFN β-1b-E, interferon β-1b Extavia®; NAT, natalizumab; PEG IFN β-1a, peginterferon β-1a.
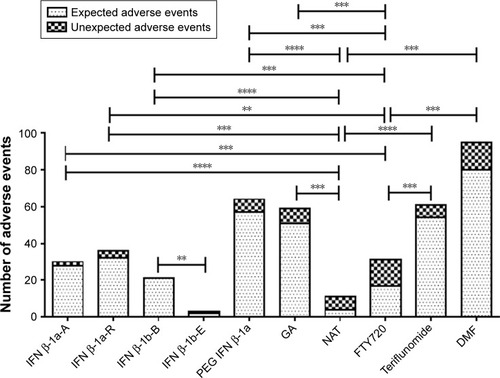
Table 2 Clinical data of RR-MS patients and adverse event characteristics for all the reported expected reactions
Table 3 Clinical data of RR-MS patients and adverse event characteristics for all the reported unexpected reactions
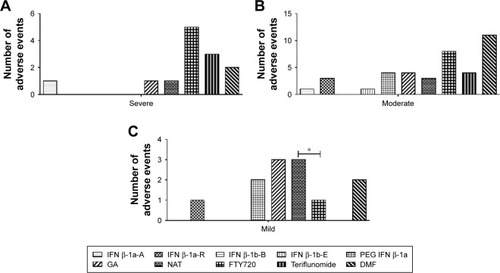
Figure 4 (A–C) Expected adverse events divided on the bases of the severity grade.
Abbreviations: DMF, dimethyl fumarate; FTY720, fingolimod; GA, glatiramer acetate; IFN β-1a-A, interferon β-1a Avonex®; IFN β-1a-R, interferon β-1a Rebif®; IFN β-1b-B, interferon β-1b Betaferon®; IFN β-1b-E, interferon β-1b Extavia®; NAT, natalizumab; PEG IFN β-1a, peginterferon β-1a.
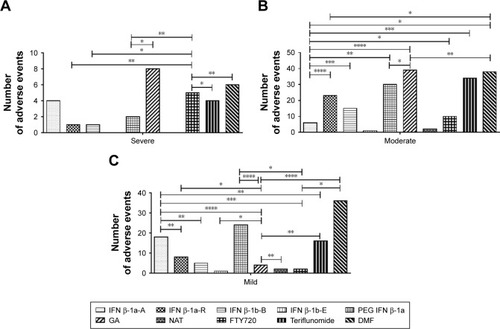
Figure 5 (A–C) Unexpected adverse events divided on the bases of the severity grade.
Abbreviations: DMF, dimethyl fumarate; FTY720, fingolimod; GA, glatiramer acetate; IFN β-1a-A, interferon β-1a Avonex®; IFN β-1a-R, interferon β-1a Rebif®; IFN β-1b-B, interferon β-1b Betaferon®; IFN β-1b-E, interferon β-1b Extavia®; NAT, natalizumab; PEG IFN β-1a, peginterferon β-1a.