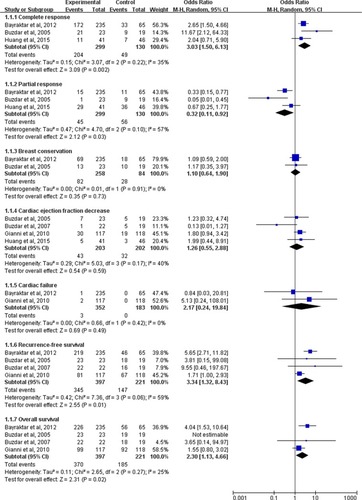
Figures & data
Figure 2 The pooled absolute rate of pCR for the concurrent (A) and nonconcurrent (B) use of trastuzumab and anthracycline-based NAC for HER2-positive breast cancer.
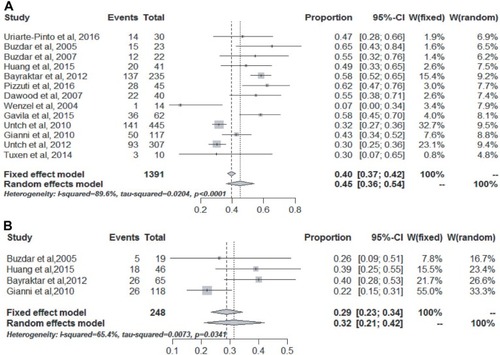
Figure 3 The pooled OR of the pCR for the comparison of the concurrent vs nonconcurrent use of trastuzumab and anthracycline-based NAC for HER2-positive breast cancer.
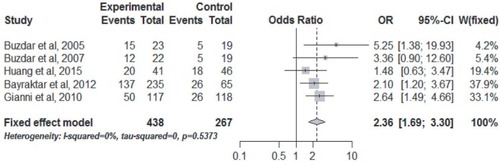
Figure 4 The pooled OR of secondary outcomes for the comparison of the concurrent vs nonconcurrent use of trastuzumab and anthracycline-based NAC for HER2-positive breast cancer.