Figures & data
Figure 1 Numbers of ADE reports submitted by SAHZU to China’s SRS and proportion of “new” and “serious” ADE reports.
Abbreviations: ADE, adverse drug event; SAHZU, the Second Affiliated Hospital of Zhejiang University; SRS, spontaneous reporting system.
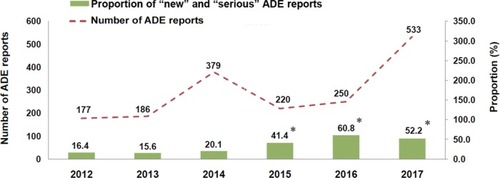
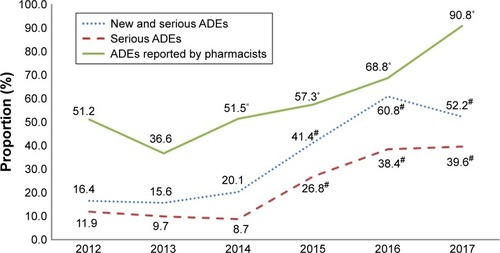
Figure 2 Proportions of “new” and “serious” ADE reports and “serious” ADE reports submitted by SAHZU to China’s SRS and the proportion of reports submitted by pharmacists during 2012–2017.
Abbreviations: ADE, adverse drug event; SAHZU, the Second Affiliated Hospital of Zhejiang University; SRS, spontaneous reporting system.