Figures & data
Figure 1 Flowchart of participants in the study.
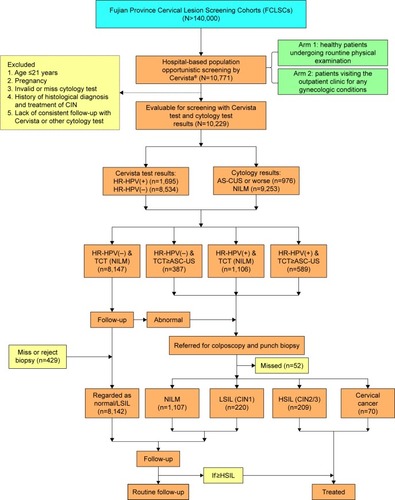
Figure 2 Prevalence of different HR-HPV infection styles and patients with different pathological results.
Abbreviations: CIN, cervical intraepithelial neoplasia; HR-HPV, high-risk human papillomavirus; NILM, negative for intraepithelial lesion or malignancy; SI, simple infection; DI, double infection; TI, triple infection.
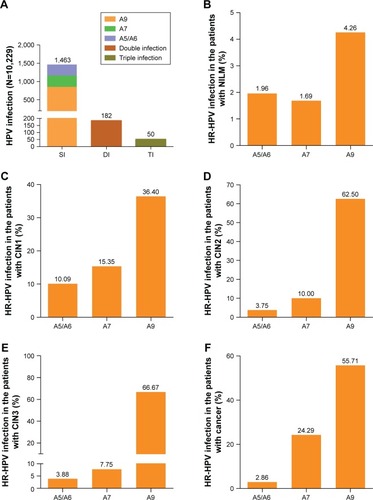
Table 1 The distribution of HR-HPV infection in different cytology results (N=10,229) (n (%))
Table 2 Compare HR-HPV infection and cytology with pathological diagnosis in 9,748 patients
Figure 3 Prevalence of HR-HPV infection and a cervical lesion in different age stratification.
Abbreviations: CIN, cervical intraepithelial neoplasia; HR-HPV, high-risk human papillomavirus.
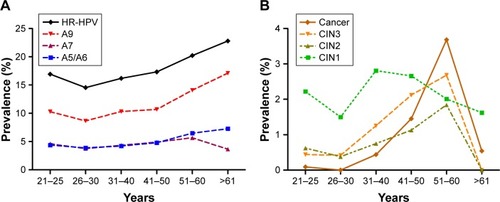
Figure 4 Combining Cervista® with TCT to detect CIN2+/CIN3+.
Abbreviations: CIN2+, cervical intraepithelial neoplasia 2 or worse; CIN3+, cervical intraepithelial neoplasia 3 or worse
Table 3 Comparing cytology and HR-HPV with co-testing in different degree of the cervical lesion (N=9,748)
