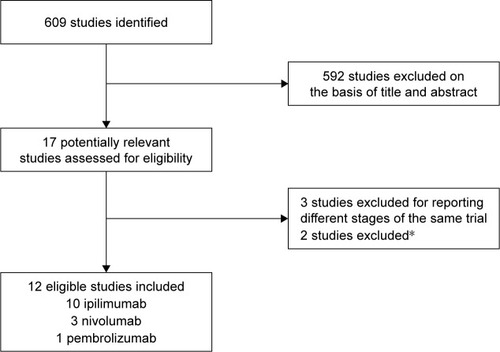
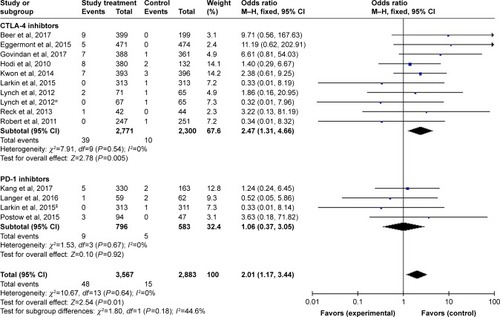
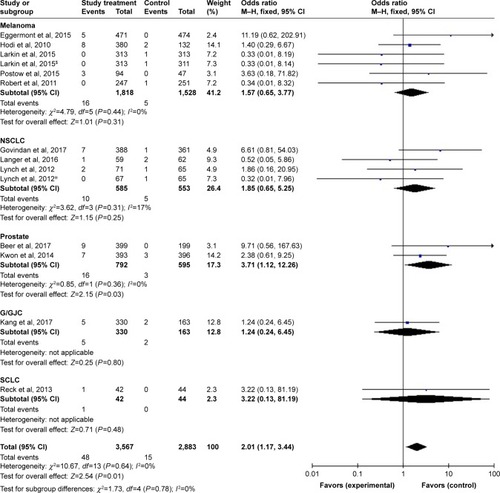
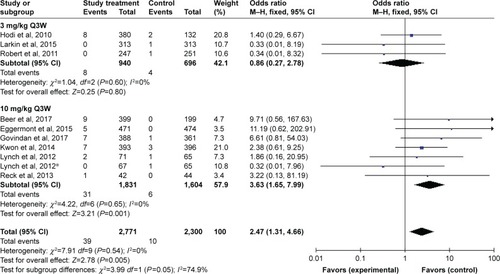
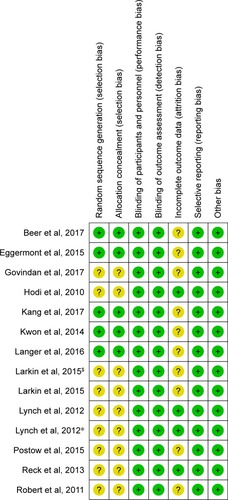
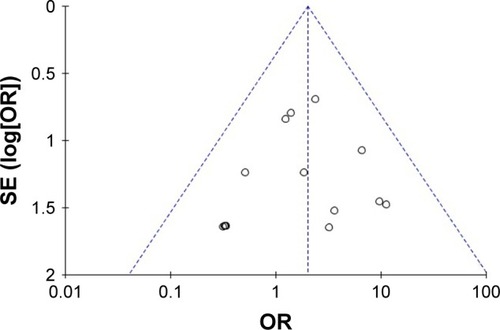
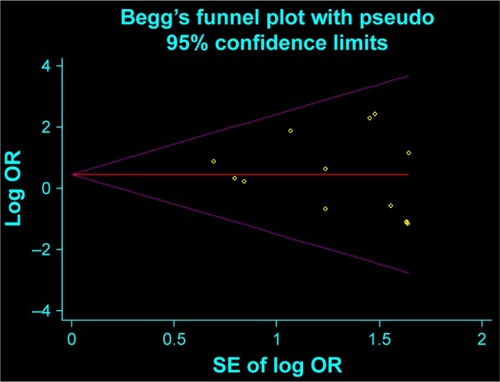
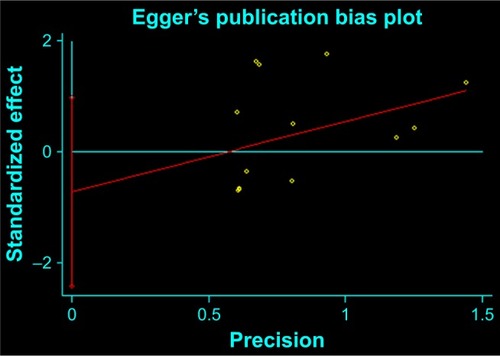
Figures & data
Table 1 Baseline characteristics of the included studies
Table 2 Characteristics of FAE type