Figures & data
Figure 1 Flow diagram of morbidly obese patients’ recruitment.
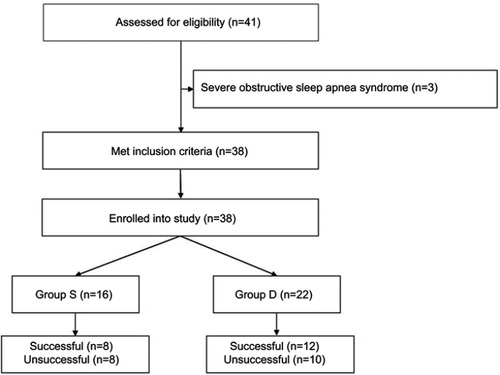
Table 1 Demographic data
Figure 2 Responses of morbidly obese patients in the two groups with the modified Dixon’s up-and-down method. Responses of 16 (group S) and 22 (group D) consecutive morbidly obese patients to BlockBusterTM SAD insertion and their ETsev with a modified Dixon’s up-and-down method are shown. Arrows indicate the mid-point dose of all independent pairs of patients who manifested cross-over from “movement” (○) to “no movement” (●) response.
Abbreviations: D, dexmedetomidine; S, saline; SAD, supraglottic airway device.

Figure 3 Dose–response curves of sevoflurane for successful insertion of BlockBusterTM SAD in the two groups. The curves plotted from probit regression analysis of individual ETsev and the responses to BlockBusterTM SAD insertion in the morbidly obese patients were shown.
Notes: Group S, saline was given intravenously; group D, a bolus dose of DEX 1 μg/kg was administered intravenously over 10 mins, followed by intravenous DEX infusion at a rate of 0.5 μg/kg/h.
Abbreviations: D, dexmedetomidine; S, saline; SAD, supraglottic airway device.
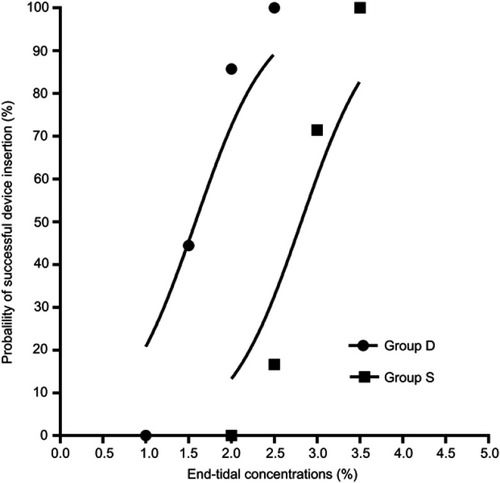
Table 2 Adverse events related to anesthetic induction and SAD insertion
