Figures & data
Figure 1 Triple mechanism of action of BIBF-1120: it inhibits all three VEGFR subtypes, PDGFR-α and PDGFR-β and FGFR types 1, 2, and 3. Other targets of this drug are the FLT-3 (inhibition of acute myelogenous leukemia cell proliferation), and members of the Src-family (Src, Lyn, and Lck).
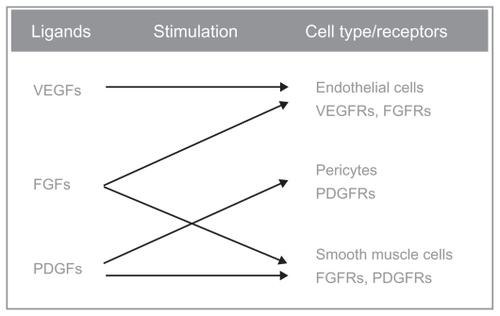
Table 1 Kinase inhibition: comparing BIBF 1120 with other small-molecule VEGFR inhibitors
Figure 2 Demographic distribution of population from a Phase II randomized double-blind study with BIBF 1120 as monotherapy in advanced non-small cell lung cancer.
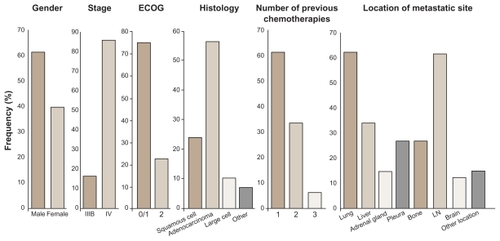
Figure 3 Demographic distribution by overall clinical response status from Phase II randomized double-blind study with BIBF 1120 as monotherapy in advanced non-small cell lung cancer.
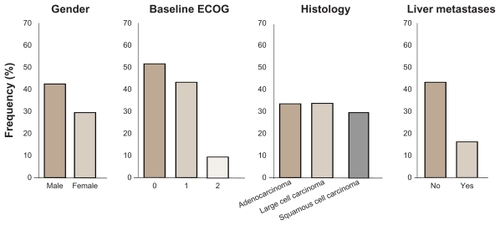
Table 2 Most frequent adverse events linked to different doses of BIBF 1120 as monotherapy from Phase II randomized doubleblind study in advanced non-small cell lung cancer