Figures & data
Figure 1 Overall study patient disposition (Consolidated Standards for Reporting Trials diagram). The study comprised 1-week, single-blind placebo run-in followed by 6-week dose-ranging study with 3 doses of PRM or placebo. Completers were allowed to continue PRM treatment for 26 weeks (N = 100) or 52 weeks (all the rest) followed by 2 weeks run-out on placebo.
Figure 2 Mean + standard error of the mean values for: (A) percentage of nights per week rated “good” or “very good” from the sleep diary during the baseline week and the last week preceding each visit during the treatment phase and withdrawal phase; and (B) percentage of days per week with mood rated “good” or “very good” from the sleep diary during the baseline week and the last week preceding each visit during the treatment phase and withdrawal phase. Blank circles indicate the values recorded at the withdrawal period following respective 26 or 52 weeks treatment with prolonged-release melatonin. The x-axis depicts the time since entering the dose ranging phase of the study. Week 1 is baseline, week 8 is the first week of the long-term period that lasted 26 weeks (ending on week 31) and 52 weeks (ending on week 59) followed by a 2-week withdrawal phase (between weeks 31–33 and 59–61 respectively).
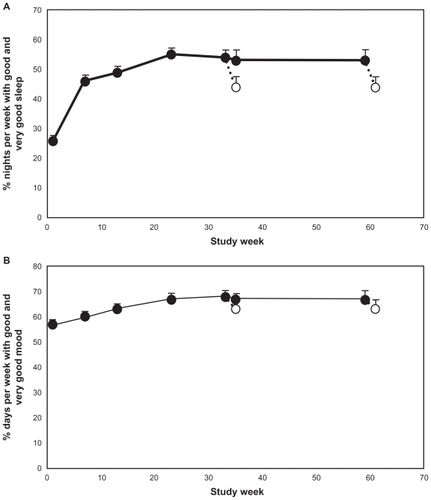
Table 1 Number of patients demonstrating a change from normal to abnormal physical examination observations between baseline and study week 33 or 59 (safety population)
Table 2 Summary of age group comparisons on adverse event incidence
Table 3 Somatic symptoms during the withdrawal period following 26 and 52 weeks of open-label PRM treatmentTable Footnotea
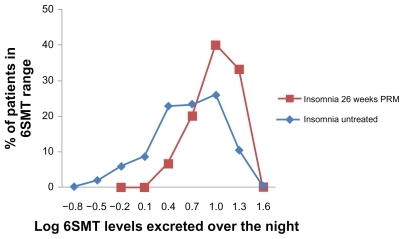
Figure 3 Frequency distribution (in percentage of patients) of 6SMT excreted over the night by patients completing 26 weeks of PRM treatment followed by 2 weeks of withdrawal (big squares; N = 15) compared with that in a historical sample 17,19 of untreated insomnia patients of the same age group (small squares; N = 384).