Figures & data
Table 1 Baseline Characteristics of Patients, According to Treatment Choices
Table 2 Baseline Characteristics After Propensity-Score Matching
Figure 1 Kaplan–Meier curves for progression-free survival before and after matching. (A) For patients before matching. (B) For patients after matching.
Abbreviations: FUL, fulvestrant; EVE-EXE, everolimus plus exemestane; HR, hazard ratio; CI, confidence interval.
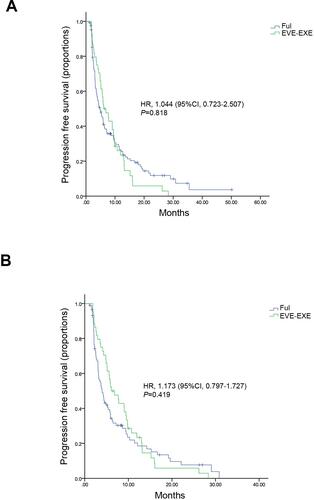
Figure 2 Forest plot of progression-free survival in FUL and EVE-EXE arms after matching.
Abbreviations: FUL, fulvestrant; EVE-EXE, everolimus plus exemestane; DFI, disease-free survival; HR, hazard ratio; CI, confidence interval.
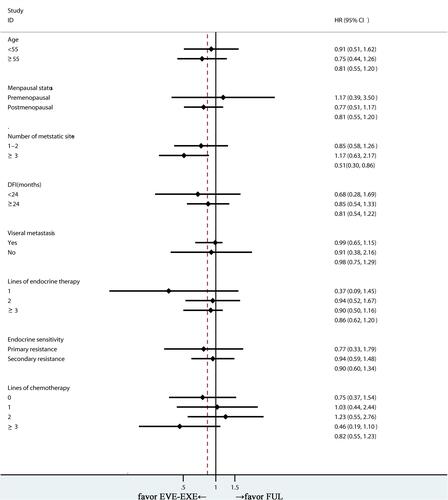
Figure 3 Kaplan–Meier curves for progression-free survival in multiple metastatic sites subgroup.
Abbreviations: FUL, fulvestrant; EVE-EXE, everolimus plus exemestane; HR, hazard ratio; CI, confidence interval.
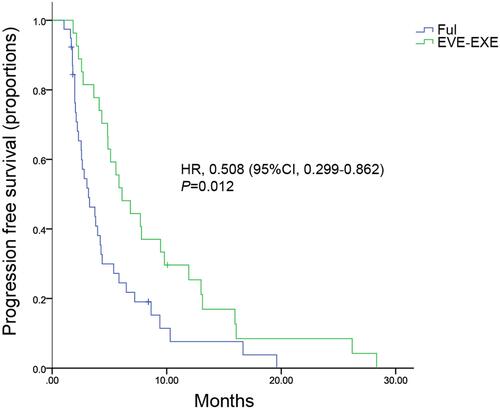
Table 3 Drug-Related AEs
