Figures & data
Figure 1 Pathophysiology of angiotensin-converting enzyme inhibitor-induced cough.Citation71,Citation72
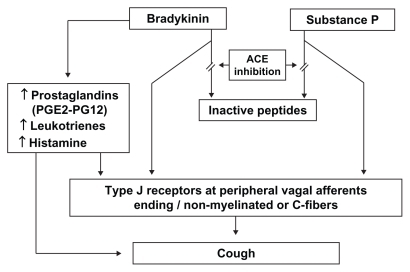
Table 1 Details of placebo or active drug controlled studies in which occurrence of cough with zofenopril was assessed in hypertensive and postmyocardial infarction patients
Figure 2 Prevalence (%) of cough under zofenopril in hypertensive patients (A) according to study design, age, and gender and (B) versus other drugs, including angiotensin II antagonists, other angiotensin-converting enzyme inhibitors, beta-blockers, and combination of zofenopril with hydrochlorothiazide.
Abbreviations: ACE, angiotensin-converting enzyme; HCTZ, hydrochlorothiazide.
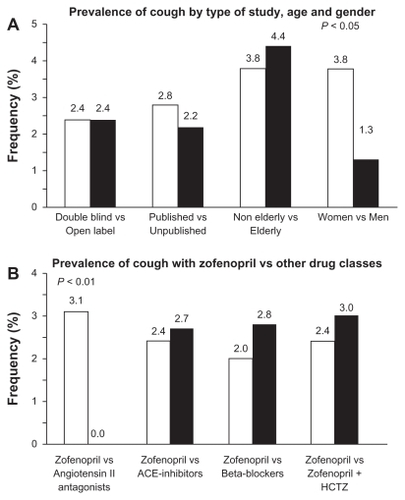
Figure 3 Incidence (%) of drug-related cough stratified by observation period during zofenopril treatment of 5794 hypertensive patients.
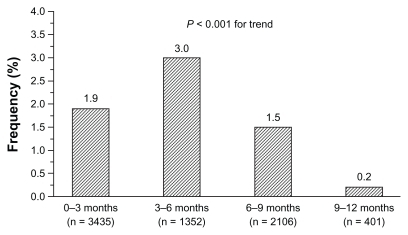
Figure 4 Prevalence (%) of drug-related cough by zofenopril dose in hypertensive patients.
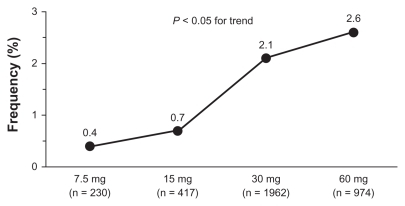
Figure 5 Rate (%) of patients with zofenopril-related cough in the postinfarction Survival of Myocardial Infarction Long-term Evaluation (SMILE) studies.Citation5–Citation7
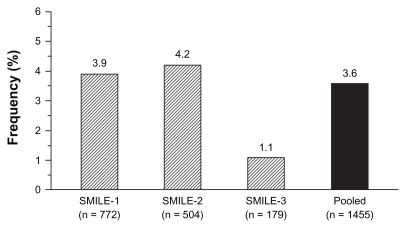
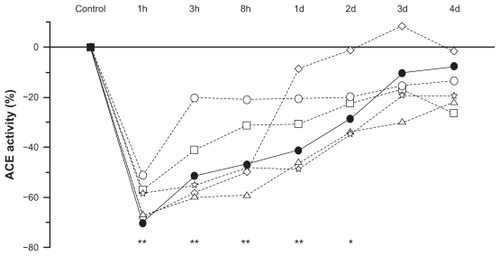
Figure 6 Changes (%) in the inhibition of lung angiotensin-converting enzyme activity as a function of time (hours and days) after administration of equivalent oral doses of zofenopril (full circles), captopril (open circles), enalapril (open squares), ramipril (open triangles), lisinopril (open stars), and fosinopril (open diamonds) in 42 rats (ex vivo study).
Abbreviations: ACE, angiotensin-converting enzyme; d, days; h, hours.