Figures & data
Figure 1 Mechanism of action of opioids on central sensitization. The dorsal root ganglion (DRG) is a group of cell bodies responsible for pain transmission from the primary afferent fibers (PAF), also known as nociceptors, and the central nervous system (CNS). Presynaptic neurons release glutamate, substance P, and calcitonin gene-related protein (CGRP) in the synaptic cleft. On the post-synaptic neurons, also named second order neurons, glutamate activate two main receptors: the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) and N-methyl-D-aspartate (NMDA) receptor. The flow of positive charged ions, calcium (Ca2+) and sodium (Na+), through the NMDA and the AMPA receptors respectively, automatically leads pain signal to increase. Substance P, on the other hand, binds to the neurokinin-1 (NK-1) receptors, which leads to intracellular signaling that activate protein kinase C (PKC). This action removes the magnesium ion (Mg2+) that physiologically blocks NMDA receptors; therefore, substance P indirectly activates NMDA receptors and increases Ca2+ influx in the neurons, leading to increased neurotransmitter release. CGRP binds specific CGRP receptors on the second order neurons, leading to a change in receptor expression and function. All these mechanisms potentially contribute to central sensitization in chronic pain. Endogenous opioids and exogenous opioid agonists bind μ opioid receptors (MOR) in the pre- and post-synaptic neurons. Opioids close the voltage-gated calcium channels on the PAF and stop Ca2+ influx in the pre-synaptic neurons, where they reduce the release of glutamate, substance P, and CGRP. In addition to that, binding of MOR on the post-synaptic neurons activate and open potassium channels, leading to an outflow of potassium ions (K+) and cellular hyperpolarization. Hyperpolarized second order neurons become less sensitive to excitatory inputs.
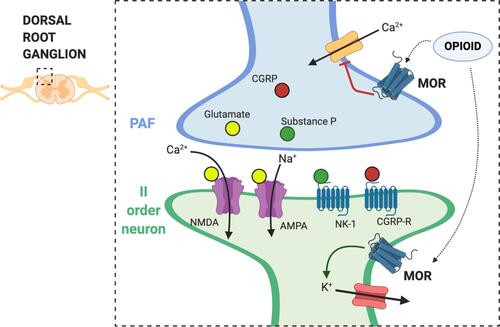
Table 1 Pharmacology of Opioids for Chronic Pain ManagementCitation39,Citation40
Table 2 Clinical Use of Opioids for Chronic Pain Management in CKD and HD PatientsCitation28,Citation30,Citation39
Table 3 Safe and Appropriate Use of Opioids in Chronic Pain ManagementCitation87
Table 4 Peripherally Acting μ-Opioid Receptor Antagonist (PAMORAs) in Patients with CKD
