Figures & data
Table 1 Overview of Primary Vibegron Studies
Table 2 Overview of Secondary, Post Hoc, and Subgroup Analyses of EMPOWUR
Figure 1 LS mean change from baseline in average daily number of (A) micturitions and (B) UUI episodes over 12 weeks. *P < 0.05. **P < 0.01. ***P < 0.001 vs placebo. Reprinted with permission from Wolters Kluwer Health, Inc.: Staskin D, Frankel J, Varano S, Shortino D, Jankowich R, Mudd PN, Jr. International phase III, randomized, double-blind, placebo and active controlled study to evaluate the safety and efficacy of vibegron in patients with symptoms of overactive bladder: EMPOWUR. J Urol. 2020;204(2):316–324. Available from: https://www.auajournals.org/doi/10.1097/JU.0000000000000807.16
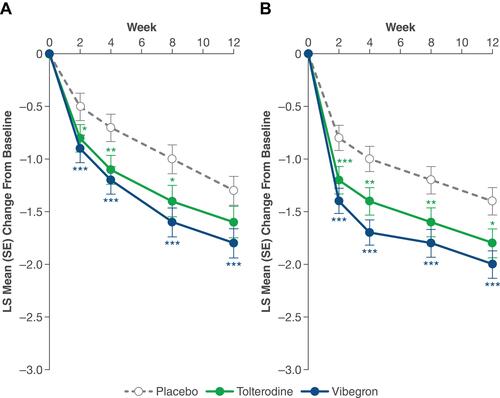
Table 3 Key Patient Baseline Demographics and Clinical Characteristics of Patients ≥65 Years Old (a Subpopulation Analysis from the EMPOWUR Trial)
Figure 2 LS mean change from baseline in average daily number of UUI episodes over 52 weeks. *P < 0.05. Reprinted with permission from Wolters Kluwer Health, Inc.: Staskin D, Frankel J, Varano S, Shortino D, Jankowich R, Mudd PN, Jr. Once-daily vibegron 75 mg for overactive bladder: long-term safety and efficacy from a double-blind extension study of the international phase 3 trial (EMPOWUR). J Urol. 2021;205(5):1421–1429. Available from: https://www.auajournals.org/doi/10.1097/JU.0000000000001574.30
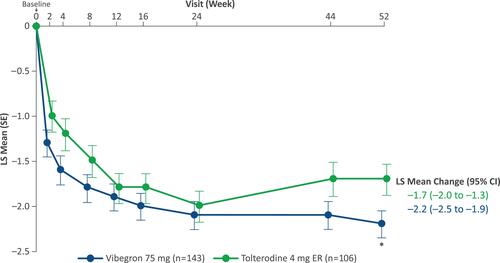
Figure 3 LS mean change from baseline in average daily number of (A) urgency episodes and (B) total urinary incontinence episodes over 52 weeks. *P < 0.05. Reprinted with permission from Wolters Kluwer Health, Inc.: Staskin D, Frankel J, Varano S, Shortino D, Jankowich R, Mudd PN, Jr. Once-daily vibegron 75 mg for overactive bladder: long-term safety and efficacy from a double-blind extension study of the international phase 3 trial (EMPOWUR). J Urol. 2021;205(5):1421–1429. Available from: https://www.auajournals.org/doi/10.1097/JU.0000000000001574.30
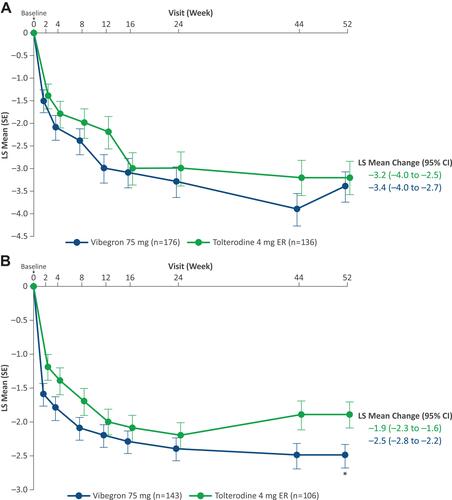
Table 4 Adverse Events by Treatment Group in the EMPOWUR Trial (Safety Analysis Set)
Table 5 Adverse Events by Treatment Group in the EMPOWUR Extension Trial (Safety Set Extension)
