Figures & data
Figure 1 Flow-chart reporting study population identification and derived cohorts: (A) study on the overall inclusion period; (B) study on the inclusion period divided into before and after 2017.
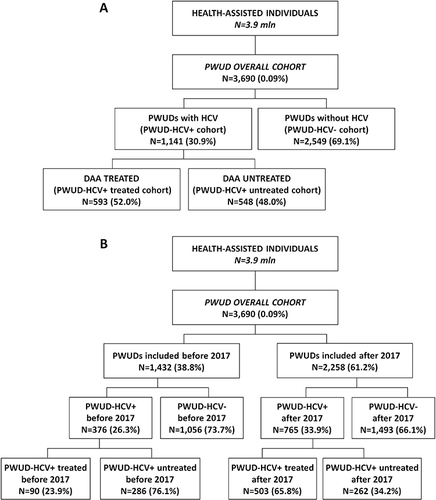
Table 1 Baseline Characteristics of PWUD Population, Overall and Stratified in PWUD-HCV+ and PWUD-HCV
Table 2 Baseline Characteristics of PWUD-HCV+ Patients, treated and Untreated with DAA-Anti HCV Medications
Table 3 Main Causes of Previous Hospitalization in PWUDs Stratified According to the Presence/Absence of HCV and, Among HCV+, to the Presence/Absence of DAA Treatment
Table 4 Number of Drugs Prescribed (Excluding DAAs) During the First Year of Follow-Up (Index-Date Included); (A) Overall PWUDs and in Patients Stratified by the HCV Diagnosis; (B) Overall PWUD-HCV+ Population, DAA-Treated and Untreated Patients
Figure 2 Treatment evaluation in overall PWUD patients and in those stratified by the HCV co-diagnosis during the first year of follow-up: distribution according to the drug ATC first level identification code (excluding DAAs).
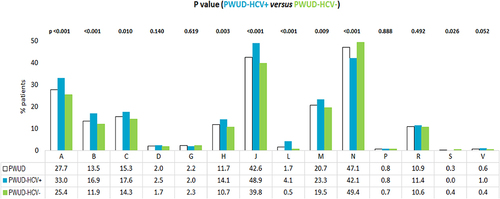
Figure 3 Treatments evaluation in overall PWUD patients and in those stratified by the HCV co-diagnosis during the first year of follow-up: distribution according to the drug ATC second level identification code (excluding DAAs).
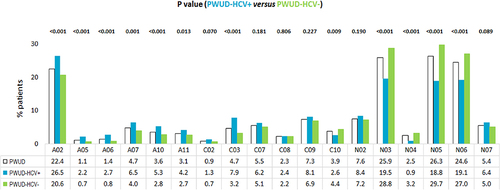
Table 5 Analysis of Healthcare Resources Consumption During First Year of Follow-Up Period
