Figures & data
Figure 1 Beneficial effects of saxagliptin on PG (A), IRI (B), CPR (C), and glucagon (D) in 75 g oral glucose tolerance test for an obese patient with types 2 diabetes mellitus.
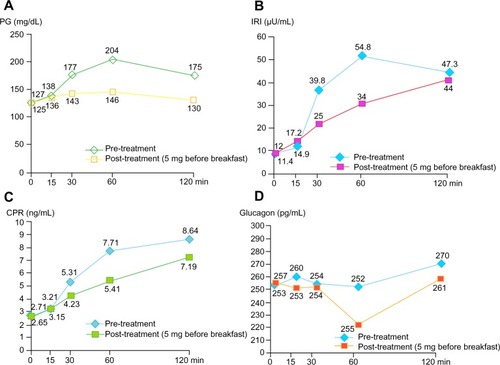
Figure 2 Mean change of HbA1c (last observation carried forward) in multiple dose study. (A) Adjusted mean change in HbA1c over time and (B) adjusted mean change in HbA1c from baseline to 12 weeks.
Abbreviations: HbA1c, glycated hemoglobin; SE, standard error of the mean; NGSP, National Glycohemoglobin Standardization Program.
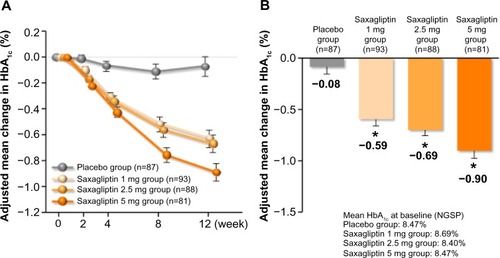
Table 1 Baseline patient characteristics in multiple dose trial
Table 2 Patient use of oral hypoglycemic agents before saxagliptin treatment in multiple dose trial
Figure 3 Adjusted mean change in HbA1c over time in saxagliptin 5 mg long-term monotherapy trial.
Abbreviations: HbA1c, glycated hemoglobin; SE, standard error of the mean; wk, weeks.
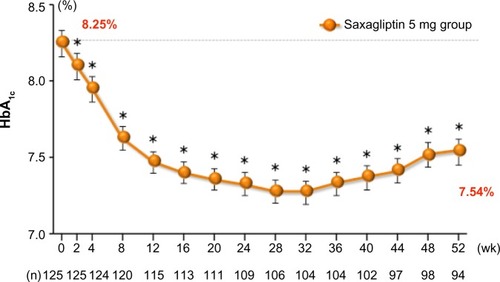
Table 3 Baseline patient characteristics in saxagliptin 5 mg long-term monotherapy trial
Table 4 Baseline patient characteristics in saxagliptin 5 mg long-term combination therapy trials
Figure 4 Changes of HbA1c in saxagliptin 5 mg long-term combination therapy trial (A) SUs CTG, (B) α-GIs CTG, (C) biguanides CTG, (D) TZDs CTG, and (E) glinides CTG.
Abbreviations: CTG, combination therapy group; SUs, sulfonylureas; TZDs, thiazolidinediones; α-GIs, α-glycosidase inhibitors; SE, standard error of the mean; HbA1c, glycated hemoglobin; wk, weeks.
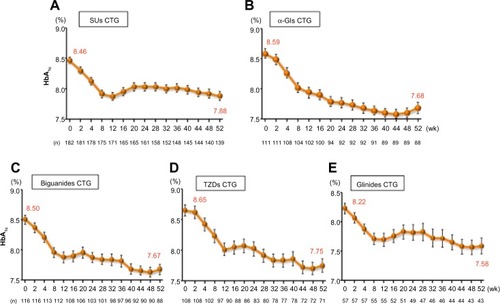
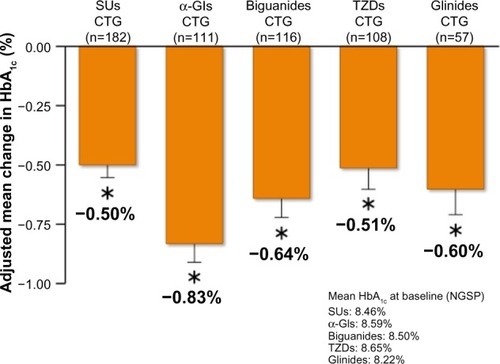
Figure 5 Adjusted mean change in HbA1c from baseline to 52 week in saxagliptin 5 mg long-term combination therapy trial.
Abbreviations: CTG, combination therapy group; SUs, sulfonylureas; TZDs, thiazolidinediones; α-GIs, α-glycosidase inhibitors; SE, standard error of the mean; HbA1c, glycated hemoglobin; NGSP, National Glycohemoglobin Standardization Program.