Figures & data
Figure 2 PILLAR study design and results. SVR rates were measured at 24 weeks after the end of treatment.
Abbreviations: PILLAR, Protease Inhibitor TMC435 Trial Assessing the Optimal Dose and Duration as Once-Daily Antiviral Regimen; PR, peginterferon plus ribavirin; RGT, response-guided therapy; SMV, simeprevir; SVR, sustained virologic response.
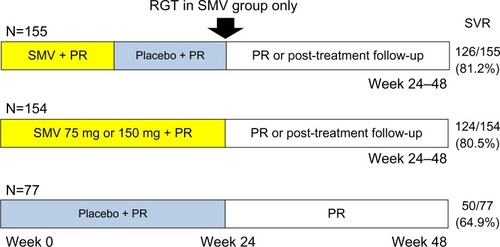
Figure 3 DRAGON study designs and results.
Abbreviations: DRAGON, A Phase II Study of TMC435 in Patients with Chronic Hepatitis C; PR, peginterferon plus ribavirin; RGT, response-guided therapy; SMV, simeprevir; SVR, sustained virologic response.
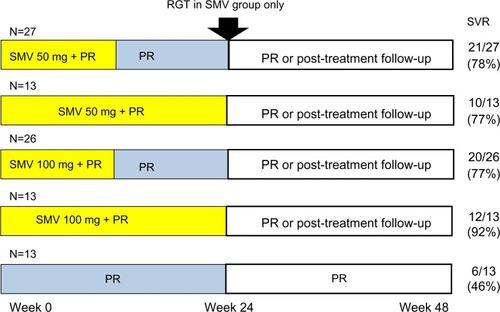
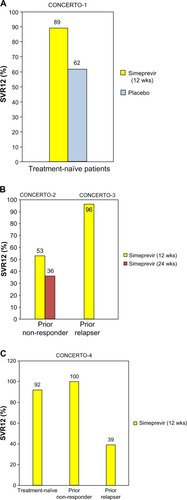
Figure 4 SVR12 in four Phase III Japanese hepatitis C virus genotype 1 patients treated with (A and B) simeprevir and ribavirin plus peginterferon α-2a or (C) peginterferon α-2b. (A) CONCERTO-1 trials for treatment-naïve patients; (B) CONCERTO-2 and CONCERTO-3 trials for prior non-responders and prior relapsers, respectively; and (C) CONCERTO-4 trials for treatment-naïve patients, prior non-responders, and prior relapsers. Data from Hayashi et al,Citation25 Izumi et al,Citation26 and Suzuki et al.Citation27