Figures & data
Figure 1 The efficacy outcome at the end of 6 weeks of treatment of acutely depressed type I bipolar patients treated with placebo, or monotherapy with lurasidone 20–60 mg/day, or lurasidone 80–120 mg/day.
Abbreviation: MADRS, Montgomery–Ȧsberg Depression Rating Scale.
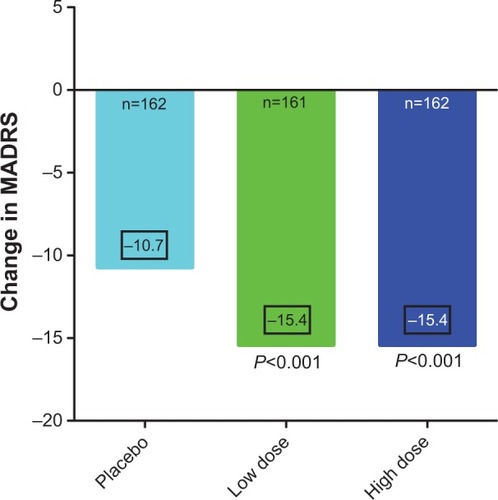
Figure 2 The efficacy outcome at the end of 6 weeks of treatment of acutely depressed type I bipolar patients treated with lithium or valproate to which either placebo was added or lurasidone 20–80 mg/day.
Abbreviations: Li, lithium; VPA, valproate; MADRS, Montgomery–Ȧsberg Depression Rating Scale.
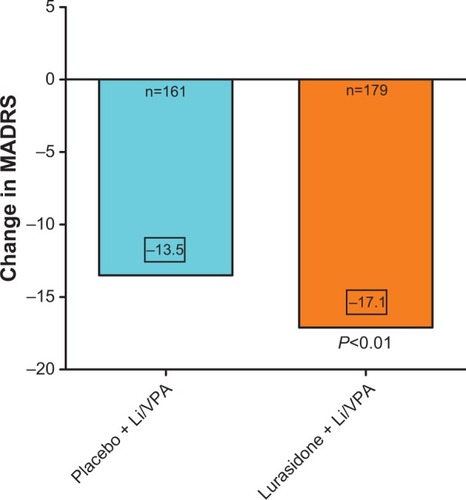
Table 1 Spontaneously reported adverse events (AEs) in the three registrational trials for lurasidone
