Figures & data
Figure 1 Forest plot of randomized clinical trials utilizing prostanoid therapies: All cause mortality. Cumulative relative risk (RR) estimate of death in active treatment groups was compared with that in control groups, excluding non-event trials. No heterogeneity was found. Fixed effect model for combined effect size was adopted.
Abbreviations: RR, relative risk; CI, confidence interval; AIR, Aerosolized Iloprost Randomized study; TRIUMPH, TReprostinil sodium Inhalation Used in the Management of Pulmonary arterial Hypertension.
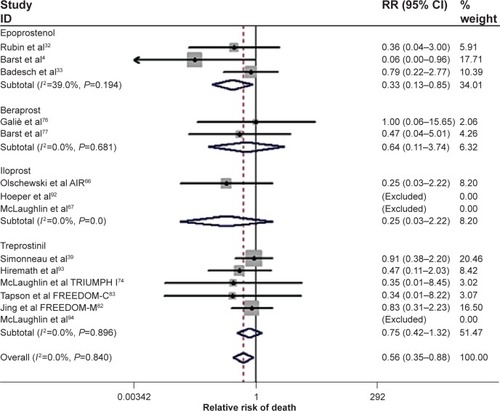
Table 1 Major randomized controlled trials of prostanoid therapy in pulmonary arterial hypertension (PAH)
