Figures & data
Figure 1 Methylnaltrexone randomized controlled trials for opioid-induced constipation treatment: study flow diagram.
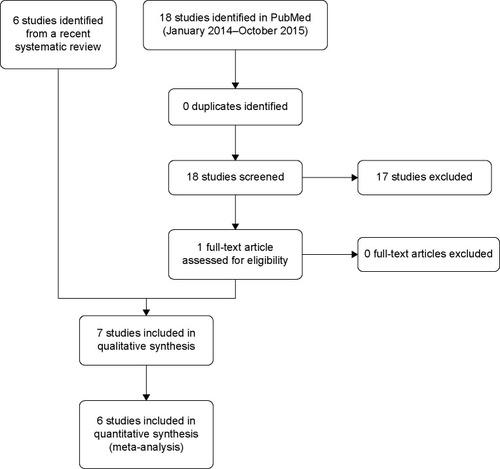
Table 1 Characteristics of included studies
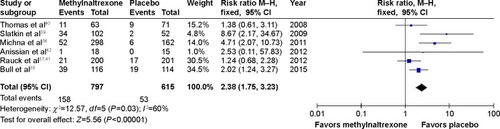
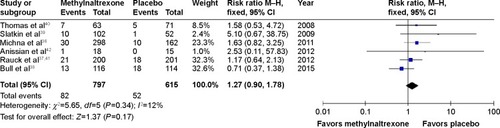
Figure 2 Methylnaltrexone versus placebo: rescue-free bowel movement within 4 hours after the first dose.
Table 2 Objective outcome measures assessing OIC
Table 3 Patient-reported outcomes assessing OIC
Table 4 Global burden measures assessing OIC
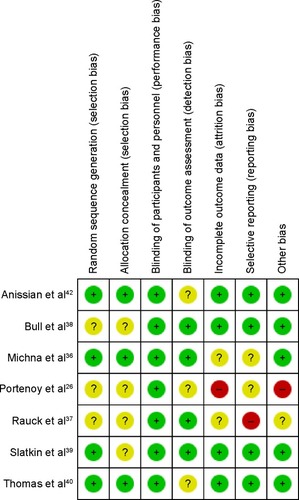
Figure 8 Risk of bias summary.