Figures & data
Figure 1 Mechanisms of action of glatiramer acetate (GA) in MS.
Reprinted from Autoimmun Rev, Vol. 6, Schrempf W, Ziemssen T, Glatiramer acetate: mechanisms of action in multiple sclerosis, 469–475.Citation9 Copyright © 2007, with permission from Elsevier. 1. GA exhibits competitive binding at the MHC-II complex and TCRantagonism. In addition, GA is able to displace MBP from the binding site on MHC-II molecules. Treatment with GA leads to the induction of antigen specific TH2 T cells in the periphery. 2. In addition CD8+ and CD4+ CD25+ regulatory T cells are induced by GA therapy. 3. The constant activation seems to have an important impact on the induction and maintenance of the regulatory/suppressive immune cells. 4. Because of the daily activation, GA T cells are believed to be able to cross the blood–brain barrier. 5. Inside the CNS, some GA-specific T cells cross-react with products of local myelin turnover presented by local APCs. 6. In response anti-inflammatory cytokines are secreted which dampen the local inflammatory process (bystander suppression). 7. Furthermore GA specific T cells secrete neurotrophic factors which might favor remyelination and axonal protection.
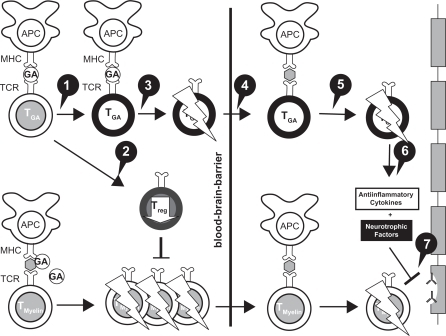
Figure 2 REGARD trial: Kaplan–Meier plot of time to first relapse.Citation14
Reprinted with permission from Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7(10):903–914. Citation14 Copyright © 2009, with permission from Elsevier.
![Figure 2 REGARD trial: Kaplan–Meier plot of time to first relapse.Citation14Reprinted with permission from Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7(10):903–914. Citation14 Copyright © 2009, with permission from Elsevier.](/cms/asset/9c4aba3c-0ff6-43a4-8f13-85238e2ed91d/dtcr_a_6743_f0002_b.jpg)
Figure 3 ARRs before and during treatment in the REGARD and BEYOND trials.
A) REGARD trial: ARRs before and during treatment.Citation14 ARR for the 2 years before and during the 96 weeks of the study in the intention-to-treat population.a Number of relapses during 96 weeks was analyzed using a Poisson regression model with factors for treatment and center. The log of time on study was used as the offset variable. B) BEYOND trial: ARRs 1 year before and during treatment. (Assessed by hazard ratios derived from generalized linear Poisson regression).Citation15 Reprinted with permission from Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7(10):903–914.Citation14 Copyright © 2009, with permission from Elsevier.
![Figure 3 ARRs before and during treatment in the REGARD and BEYOND trials.A) REGARD trial: ARRs before and during treatment.Citation14 ARR for the 2 years before and during the 96 weeks of the study in the intention-to-treat population.a Number of relapses during 96 weeks was analyzed using a Poisson regression model with factors for treatment and center. The log of time on study was used as the offset variable. B) BEYOND trial: ARRs 1 year before and during treatment. (Assessed by hazard ratios derived from generalized linear Poisson regression).Citation15 Reprinted with permission from Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7(10):903–914.Citation14 Copyright © 2009, with permission from Elsevier.](/cms/asset/1f5c07dd-5607-4195-8a89-81da3dfba50c/dtcr_a_6743_f0003_b.jpg)
Figure 4 Time to conversion to CDMS in the placebo-controlled phase. Kaplan–Meier curves with Cox’s proportional hazard ratio were used to model the amount of time to conversion to CDMS for patients assigned to GA and placebo. A delay of 386 days (115%) in conversion to CDMS was noted for first quartile of patients receiving GA compared with those receiving placebo. Reprinted from The Lancet, Comi G, Martinelli V, Rodegher M, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:1503–1511.Citation17 Copyright 2009, with permission from Elsevier.
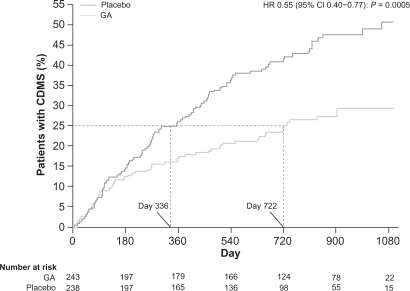
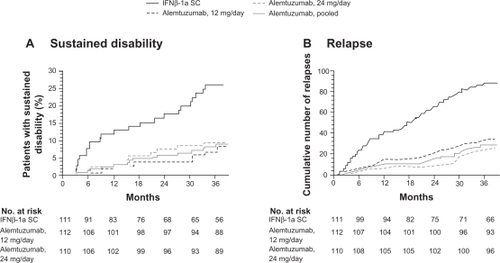
Figure 5 Primary endpoints of the CAMMS223 trial – sustained disability and relapse.
A) Kaplan–Meier curves for patients who reached the criteria for sustained accumulation of disability. B) Cumulative number of relapses. Reprinted with permission from CAMMS223 Trial Investigators, Coles AJ, Compston DA, et al. Alemtuzumab vs interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786–1801.Citation73 Copyright ® 2009 Massachusetts Medical Society. All rights reserved.