Figures & data
Figure 1 Mean change in seated systolic blood pressure (SeSBP) from baseline to weeks 2, 4, 6 and 8 with olmesartan (OLM) and amlodipine (AML) monotherapy and olmesartan/amlodipine combination therapy.
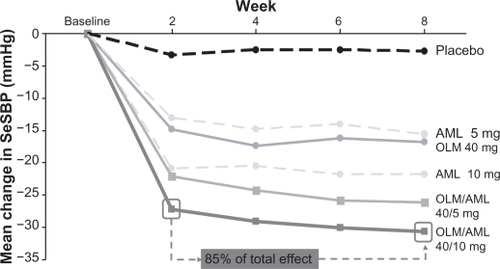
Table 1 The COACH trial – change in seated diastolic blood pressure (SeDBP) and seated systolic blood pressure (SeSBP) from baseline to week 8 in the intent-to-treat population (last observation carried forward)Citation46
Table 2 The COACH trial – patients achieving the blood pressure target (<140/90 mmHg for patients with uncomplicated hypertension; <130/80 mmHg for patients with diabetes) after eight weeks of treatment (last observation carried forward)Citation46
Figure 2 Olmesartan/amlodipine combination therapy versus amlodipine monotherapy – mean change from baseline in seated blood pressure after eight weeks of randomized, double-blind treatment.Citation49
Abbreviations: AML, amlodipine; OLM, olmesartan; SeBP, seated blood pressure; SeDBP, seated diastolic blood pressure; SeSBP, seated systolic blood pressure.
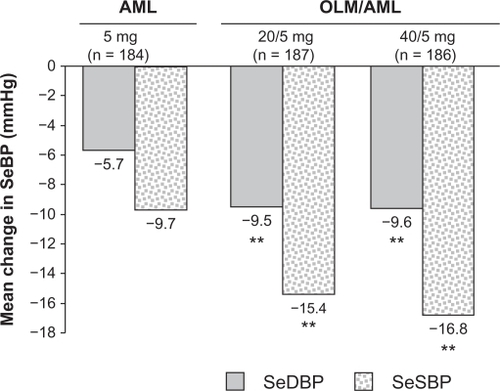
Figure 3 Olmesartan/amlodipine combination therapy versus amlodipine monotherapy – mean change from baseline in seated systolic blood pressure after eight weeks of randomized, double-blind, uptitrated treatment.Citation49
Abbreviations: AML, amlodipine; OLM, olmesartan; SeSBP, seated systolic blood pressure.
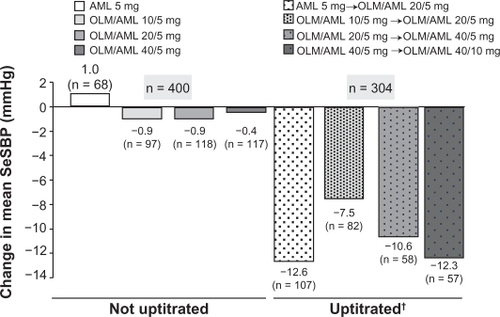
Figure 4 Mean levels of seated systolic blood pressure (SeSBP) at the start (week 0) and end of treatment (week 52) according to baseline SeSBP in all patients treated with olmesartan/amlodipine combination therapy in a randomized, double-blind study.Citation58
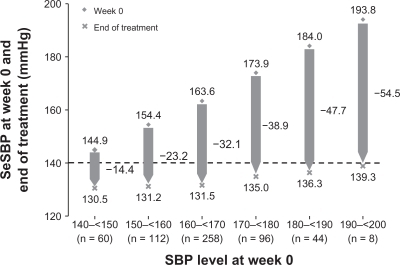
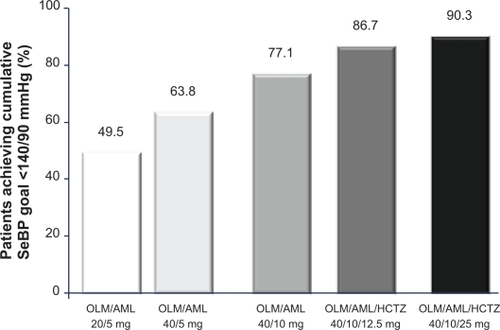
Figure 5 Proportion of patients who achieved the cumulative seated blood pressure (SeBP) goal of 140/90 mmHg in the BP-CRUSH study.Citation59