Figures & data
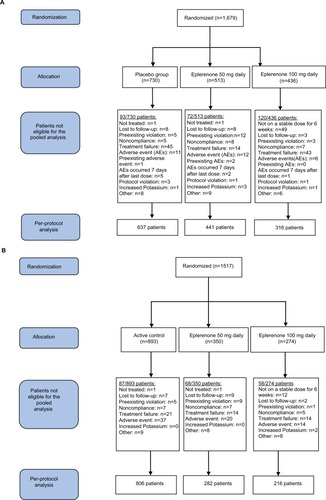
Table 1 Overview of patient disposition
Table 2 Baseline demographic and clinical characteristics of per-protocol setTable Footnotea
Table 3 Change from baseline in seated SBP/DBP
Table 4 Treatment-emergent adverse events
Figure 2 Change from baseline in seated SBP/DBP: (A) placebo-controlled studies and (B) active-controlled studies.
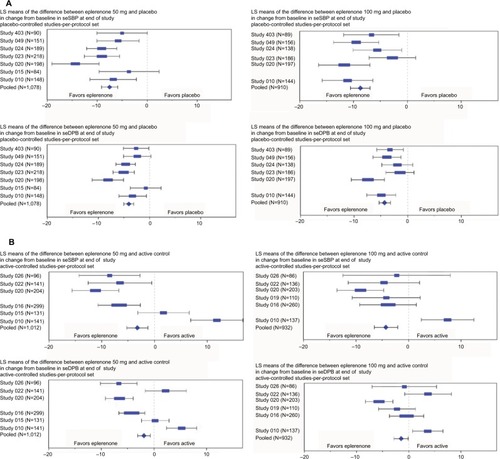
Table 5 Potassium laboratory values
Table S1 Adequate and well-controlled studies included in pooled analysis
Table S2 Other controlled studies not included in pooled analysis
Table S3 Uncontrolled studies not included in pooled analysis