Figures & data
Table 1 Ongoing clinical trials of stem-cell therapy for heart diseases
Figure 1 Types of stem cells in use for heart disease therapy.Citation1–Citation7
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2011–2012. All Rights Reserved.
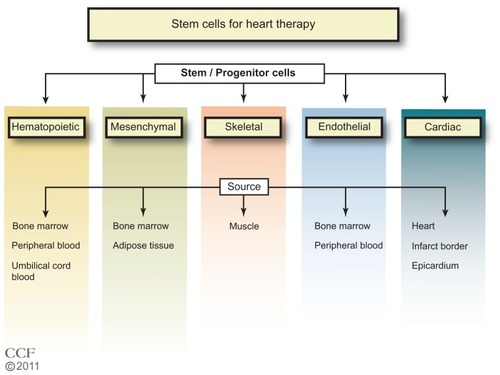
Figure 2 Expansion of stem cells.
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2011–2012. All Rights Reserved.
Abbreviations: ESC, embryonic stem cell; iPS, induced pluripotent stem; MSC, mesenchymal stem cell.
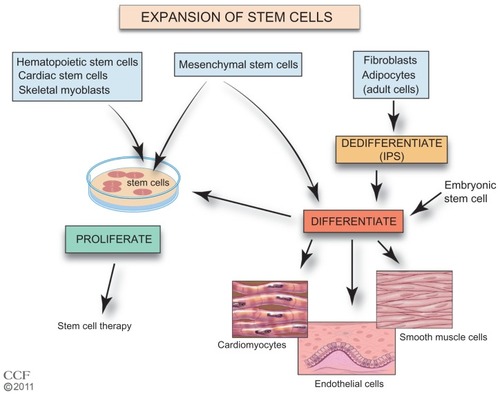
Figure 3 Stem cell mobilization and homing. Growth factors and cytokines stimulate the mobilization of the stem cells from their niche to injured tissue.
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2011–2012. All Rights Reserved.
Abbreviations: G-CSF, granulocyte colony-stimulating factor; Gm-CSF, granulocyte-macrophage colony-stimulating factor; SCF, stem cell factor/c-kit ligand; SDF-1, stromal cell-derived factor 1; MCP-3, monocyte chemotactic protein-3; GRO-1, growth regulated oncogene 1; HGF, hepatic growth factor; FGF-2, fibroblast growth factor; IGF-1, insulin-like growth factor.
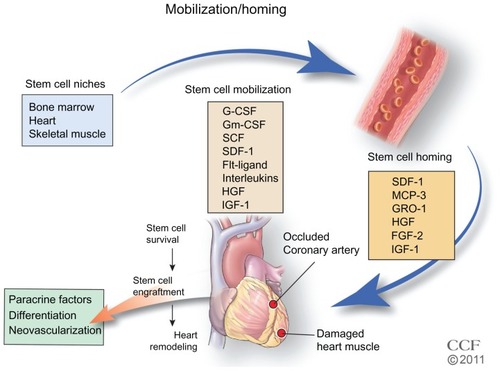
Figure 4 Bone marrow-derived stem cell mobilization. Bone marrow stem cells may be mobilized by reducing the ligand SDF-1 and increasing the stem cell receptor CXCR4 to create a chemotatic gradient with the peripheral blood. G-CSF treatment increases MMP-9 to regulate changes in SDF-1/CXCR4 pathway, which is dependent on plasmin activation of MMP-9.
Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2011–2012. All Rights Reserved.
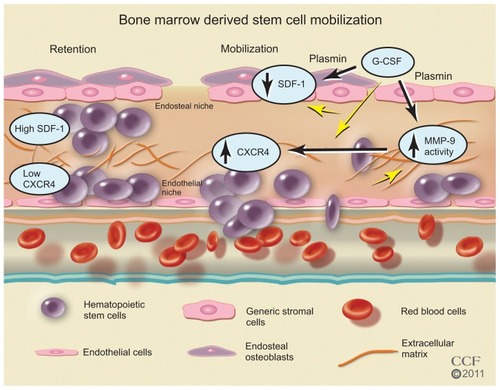
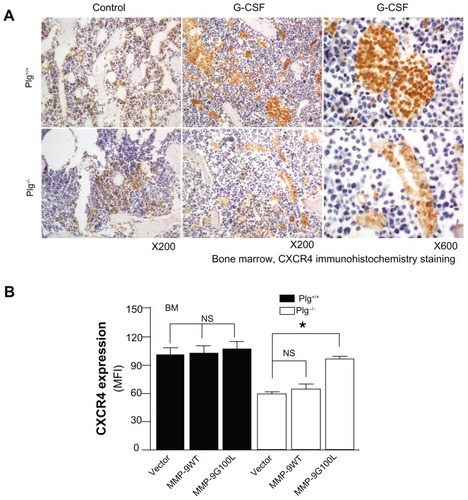
Figure 5 Plasminogen regulates CXCR4 after G-CSF stimulation. (A) CXCR4 immunostaining of bone marrow from Plg+/+ and Plg−/− mice treated with saline (control) or G-CSF. CXCR4 expressing cells (brown color) increased two fold after G-CSF treatment in Plg+/+ mice, but CXCR4 did not change in Plg−/− mice. (B) Lentivirus expression of act MMP-9 in Plg−/− restored CXCR4 expression. Plasminogen activation of MMP-9 is required for CXCR4 expression after G-CSF treatment.
Abbreviations: CXCR4, C-X-C receptor 4; G-CSF, granulocyte colony-stimulating factor; MFI, mean fluorescence intensity; MMP-9, matrix metalloproteinase-9; Plg, plasminogen.