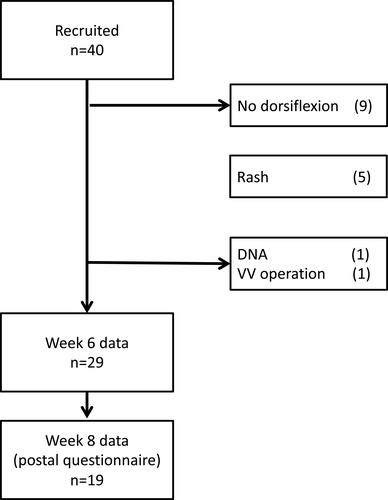
Figures & data
Table 1 Demographic Data of Recruited Subjects
Table 2 Anatomical Segments Affected by Venous Disease in Trial Patients (Disease may affect more than one segment in any one subject, therefore total not equal to participant number in each group)
Table 3 Haemodynamic changes in the femoral vein, with use of NMES, “Device off” compared to “Device on”
Figure 2 Haemodynamic changes in the femoral vein with neuromuscular electrical stimulation, taken at weeks 0 and 6 (*p<0.05, Wilcoxon matched pairs signed-rank).
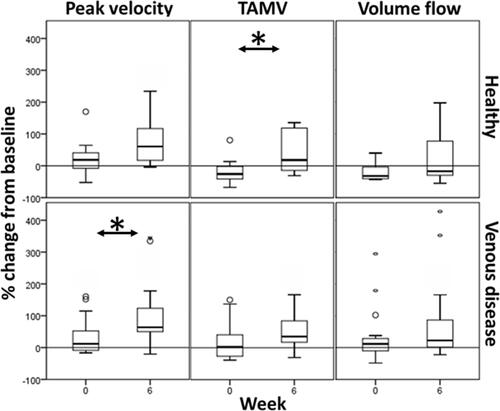
Table 4 Laser Doppler Fluximetry changes in the hand and foot with use of NMES, “Device off” compared to “Device on”
Table 5 Changes in unilateral leg volume over 6 weeks regular NMES use
Table 6 Mean Quality of Life Scores and Changes Over Time