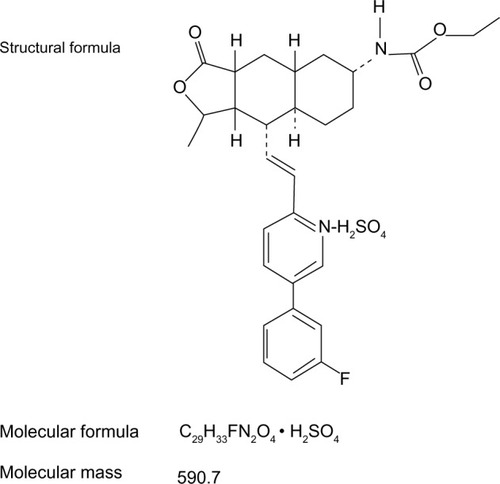
Figures & data
Figure 1 Pathways of platelet protease-activated receptor (PAR)-1 activation.
Abbreviations: GEFs, guanine nucleotide exchange factors; IP3, inositol trisphosphate 3; PI3-kinase, phosphoinositide-3 kinase; MAP, mitogen activated kinase; DAG, diacylglycerol; WASP, Wiskott–Aldrich syndrome protein; SRE, serum response element; MLC, myosin light chain; PHD, prolyl hydroxylase domain.
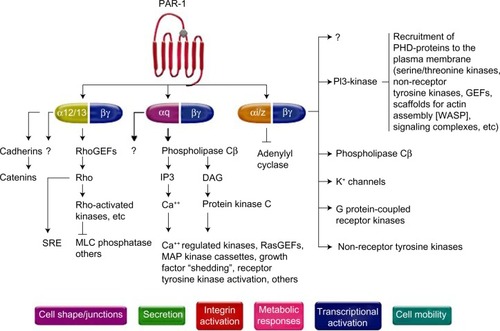
Table 1 Efficacy and safety end points in the TRACER (at 2 years) trial
Table 2 Efficacy and safety end points in the TRA 2°P – TIMI 50 (at 3 years) trial
Figure 3 Kaplan–Meier curve of estimated occurrence of cardiovascular death, myocardial infarction, or stroke in TRA 2°P – TIMI 50 prior myocardial infarction cohort.
Abbreviations: HR, hazard ratio; CI, confidence interval; TIMI, Thrombolysis in Myocardial Infarction.
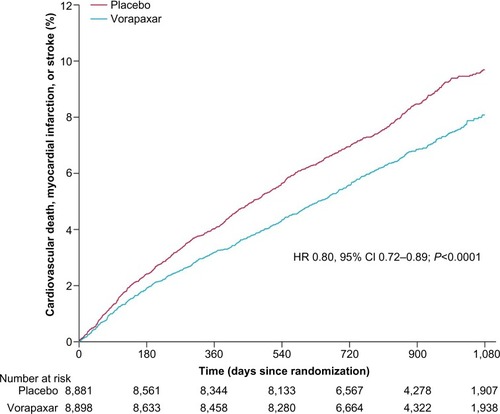
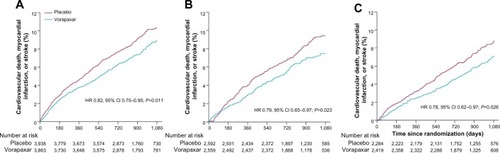
Figure 4 Kaplan–Meier estimates of cardiovascular death, myocardial infarction, or stroke according to time from qualifying myocardial infarction to randomization: <3 months (A), 3–6 months (B), and >6 months (C) in the TRA 2°P–TIMI 50 prior myocardial infarction cohort.
Abbreviations: HR, hazard ratio; CI, confidence interval; TIMI, Thrombolysis in Myocardial Infarction.