Figures & data
Figure 1 Effects of exenatide on glycosylated hemoglobin, body weight, apolipoproteins, and lipoproteins in the total analysis cohort with an overall normal lipid profile at baseline. Week 30 change data are independent of glycemic improvement and weight loss. (A) Glycosylated hemoglobin. (B) Body weight. (C) Apolipoprotein B. (D) Percentage of apolipoprotein B/apolipoprotein A1. (E) Low-density lipoprotein cholesterol and its subclasses. (F) Triglycerides, very low-density lipoprotein cholesterol, and non-high-density lipoprotein cholesterol. (G) High-density lipoprotein cholesterol and its subclasses. (H) Percentage changes in high-density lipoprotein cholesterol and its subclasses. (Panels A and B) Least squares mean + 95% confidence intervals. *Change from baseline P < 0.0001. (Panels C–G) Adjusted mean + standard error of the mean.
Abbreviations: A1c, glycosylated hemoglobin; ApoB, apolipoprotein B; ApoA1, apolipoprotein A1; BID, twice daily; LDL-C, low-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; QW, once weekly.
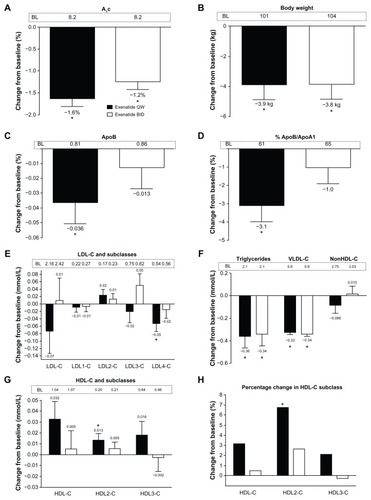
Table 1 Demographics and baseline characteristics for the lipoprotein subclass analysis cohort
Table 2 Apolipoprotein and lipoprotein change from baseline in patients with type treated with exenatide for 30 weeks for the lipoprotein subclass analysis cohort
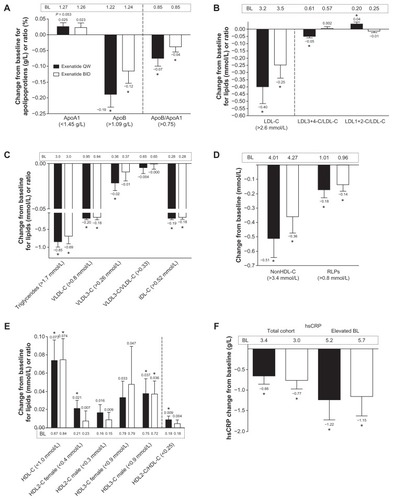
Figure 2 Effects of exenatide on apolipoproteins, lipoproteins, and high sensitivity C-reactive protein in the subgroup of patients with abnormal lipid valuesCitation1–Citation3 at baseline. (A) Apolipoprotein A1, apolipoprotein B, and the ratio of these apolipoproteins; once weekly, n = 85, n = 9, and n = 22, respectively; twice daily, n = 78, 18, and 29, respectively. (B) Low-density lipoprotein cholesterol and subclass ratios; once weekly, n = 28, 28, and 28, respectively; twice daily, n = 36, 36, and 36, respectively. (C) Triglycerides, very low-density lipoprotein (VLDL) cholesterol, VLDL3 cholesterol, ratio of VLDL cholesterol/VLDL3 cholesterol, and intermediate-density lipoprotein cholesterol; once weekly, n = 55, 18, 77, 106, and 7, respectively; twice daily, n = 52, 20, 75, 104, and 6, respectively. (D) Non-high-density lipoprotein cholesterol and remnant lipoproteins; once weekly, n = 26 and n = 13, respectively; and twice daily, n = 34 and n = 21, respectively. (E) Total high-density lipoprotein cholesterol and its subclasses stratified by gender and overall subclass ratio; once weekly, n = 51, 39, 57, 22, 47, and 95, respectively; twice daily, n = 46, 39, 55, 17, 44, and 96, respectively. (F) High sensitivity C-reactive protein in the total cohort; once weekly, n = 104; twice daily, n = 103.
Abbreviations: BL, baseline; ApoB, apolipoprotein B; ApoA1, apolipoprotein A1; LDL-C, low-density lipoprotein cholesterol; BID, twice daily; HDL-C, high-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol; IDL-C, intermediate-density lipoprotein cholesterol; RLPs, remnant lipoproteins; hsCRP, high sensitivity C-reactive protein; QW, once weekly.