Figures & data
Table 1 Early prospective randomized clinical trials of intravenous tPA in acute ischemic stroke
Figure 1 (A) Death or dependency defined as mRS 2–6. (B) Risk of symptomatic intracerebral hemorrhage.
Notes: A and B demonstrate point estimates for ORs and 95% CIs between the tPA and control groups for each of the trials. *References for the listed trials: NINDS,Citation1 ECASS,Citation20 ECASS II,Citation29 ATLANTIS-A,Citation19,Citation28 ATLANTIS-B,Citation49 and ECASS III.Citation33
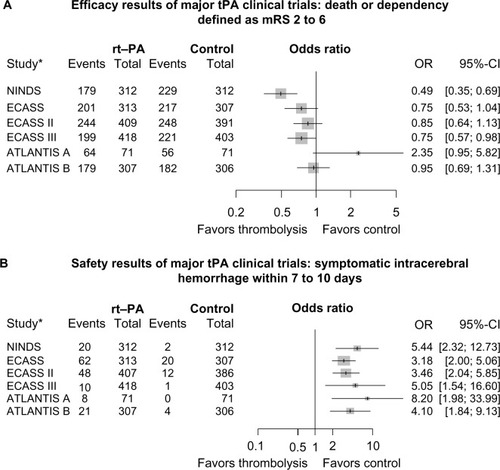
Table 2 tPA-related hemorrhage as defined by different stroke studies
Table 3 Risk factor profiles associated with negative outcomes after use of tPA in acute ischemic stroke
Table 4 Risk and prognostic stratification scales
Table 5 Common stroke mimics
Table 6 Community-based studies on the experience of tPA utilization for acute ischemic stroke
