Figures & data
Figure 1 (A) Adjusted mean (±standard error of the mean) change from baseline to 24-week endpoint in A1C in patients with T2DM adding vildagliptin 50 mg bid (closed bars; n = 221) or placebo (open bars; n = 215) to their ongoing insulin regimen (with or without metformin) and between-group difference (open bars). aP < 0.001. (B) Proportion of patients with T2DM experiencing confirmed hypoglycemic episodes during 24-week treatment with vildagliptin (50 mg bid, closed bars; n = 227) or placebo (open bars; n = 221) added to their ongoing insulin regimen (with or without metformin). (C) Mean change from baseline to 24-week endpoint in body weight in patients with T2DM adding vildagliptin 50 mg bid (closed bars; n = 222) or placebo (open bars; n = 215) to their ongoing insulin regimen (with or without metformin).Citation22
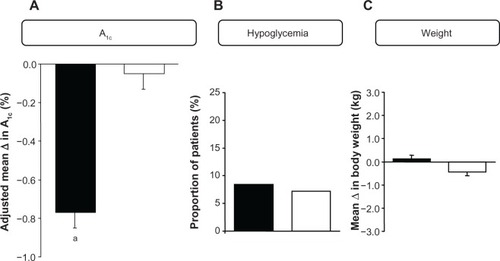
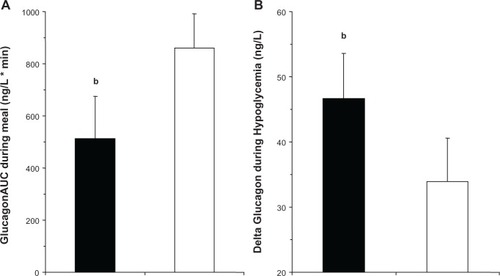
Figure 2 (A) AUC during the frst 60 minutes of a standardized mixed meal in drug-naïve patients with T2DM on day 28 of treatment with vildagliptin (100 mg qd, closed bars) or placebo (open bars) during a crossover study (n = 25 patients). bP ,< 0.05. (B) Change in plasma glucagon levels from beginning to end of the 2.5 mM glucose step of a stepped hyperinsulinemic hypoglycemic clamp performed in drug-naive patients with T2DM on day 28 of treatment with vildagliptin (100 mg qd, closed bars) or placebo (open bars) during a crossover study (n = 25 patients).Citation34
Abbreviations: AUC, area under the curve; T2DM, type 2 diabetes; qd, once daily.