Figures & data
Figure 1 The renin–angiotensin–aldosterone system (RAAS) and antihypertensive drug classes affecting various sites of the RAAS. Copyright © 2008, Informa Healthcare. All rights reserved. Adapted with permission from Rashid H. Direct renin inhibition: an evaluation of the safety and tolerability of aliskiren. Curr Med Res Opin. 2008;24:2627–2637.Citation9
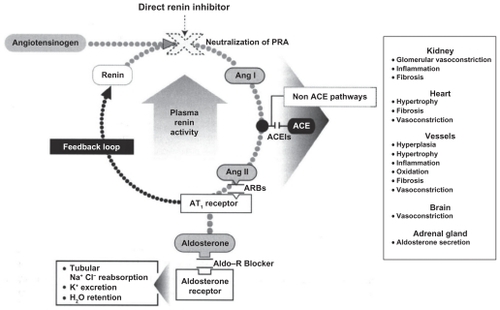
Table 1 Changes in renin, plasma renin activity, plasma Ang I, plasma Ang II and tissue Ang II levels in response to antihypertensive drug classes affecting various sites of the RAAS
Figure 2 A) Changes from baseline in 24-hour mean ambulatory systolic and diastolic blood pressure (BP) after a missed dose of aliskiren 300 mg, irbesartan 300 mg or ramipril 10 mg. Hourly change in 24-hour mean ambulatory systolic BP at baseline after a missed dose of treatment in patients receiving aliskiren 300 mg (B), irbesartan 300 mg (C) or ramipril 10 mg (D).
**P < 0.01 for pair-wise comparison [by analysis of covariance (ANCOVA)].
Copyright © 2010, Nature Publishing Group, a subsidiary of Macmillan Publishers Ltd, and Nature America Inc. All rights reserved. Adapted with permission from Palatini P, Wung W, Shlyakhto E, Botha J, Bush C, Keefe DL. Maintenance of blood-pressure lowering effect following a missed dose of aliskiren, irbesartan or ramipril: Results of a randomized, double-blind study. J Hum Hypertens. 2010;24:93–103.Citation23
![Figure 2 A) Changes from baseline in 24-hour mean ambulatory systolic and diastolic blood pressure (BP) after a missed dose of aliskiren 300 mg, irbesartan 300 mg or ramipril 10 mg. Hourly change in 24-hour mean ambulatory systolic BP at baseline after a missed dose of treatment in patients receiving aliskiren 300 mg (B), irbesartan 300 mg (C) or ramipril 10 mg (D).**P < 0.01 for pair-wise comparison [by analysis of covariance (ANCOVA)].Copyright © 2010, Nature Publishing Group, a subsidiary of Macmillan Publishers Ltd, and Nature America Inc. All rights reserved. Adapted with permission from Palatini P, Wung W, Shlyakhto E, Botha J, Bush C, Keefe DL. Maintenance of blood-pressure lowering effect following a missed dose of aliskiren, irbesartan or ramipril: Results of a randomized, double-blind study. J Hum Hypertens. 2010;24:93–103.Citation23](/cms/asset/cd38d85a-afce-4c48-9033-97b5b2a1b302/dvhr_a_4189_f0002_b.jpg)
Figure 3 Primary end-point of the ALOFT study, change in plasma BNP from baseline to three months in aliskiren- or placebo-treated chronic heart failure (CHF) patients.
Copyright © 2007, European Society of Cardiology. All rights reserved. Adapted with permission from Seed A, Gardner R, McMurray J, et al. Neurohumoral effects of the new orally active renin inhibitor, aliskiren, in chronic heart failure. Eur J Heart Fail. 2007;9:1120–1127.Citation52
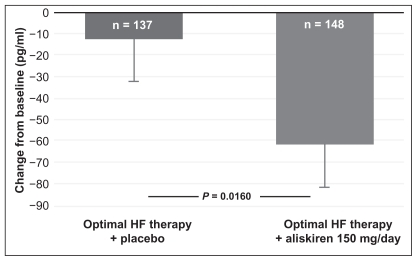
Figure 4 Primary efficacy analysis of the ALLAY Study. Comparison of left ventricular (LV) mass regression in patients receiving aliskiren, losartan or their combination. Bars show the mean ± SEM for the efficacy population.
Copyright © 2009, Wolters Kluwer Health. Adapted with permission from Solomon SD, Appelbaum E, Manning WJ, et al; Aliskiren in Left Ventricular Hypertrophy (ALLAY) Trial Investigators. Effect of the direct renin inhibitor aliskiren, the angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009;119:530–537.Citation32
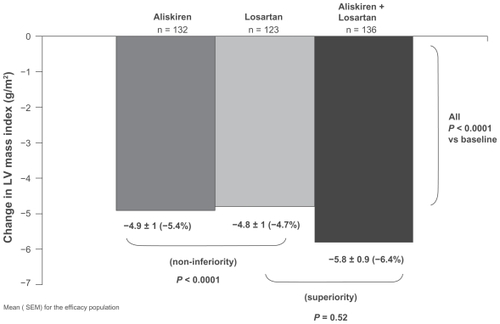
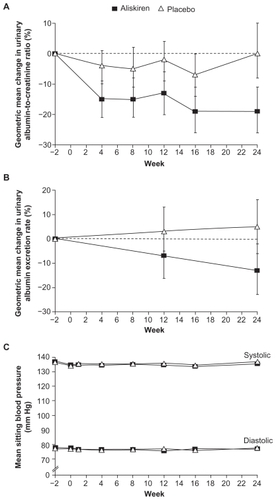
Figure 5 Changes from baseline (2 weeks before randomization) in the urinary albuminto- creatinine ratio (Panel A), urinary albumin excretion rate (Panel B) and mean sitting blood pressure (Panel C) according to Study Group in the AVOID Study.
Copyright © 2008, Massachusetts Medical Society. All rights reserved. Adapted with permission from Parving HH, Persson F, Lewis JB, Lewis EJ, Hollengerg NH. Aliskiren combined with losartan in Type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446.Citation59