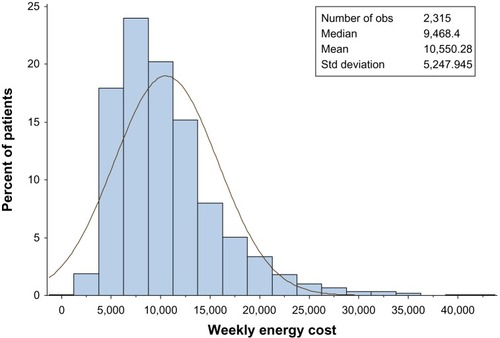
Figures & data
Table 1 Patient baseline characteristicsTable Footnote*
Table 2 Overview of adverse drug reactions or serious adverse drug reactions based on the safety set
Figure 1 Blood pressure reduction (mmHg) between study start and end.
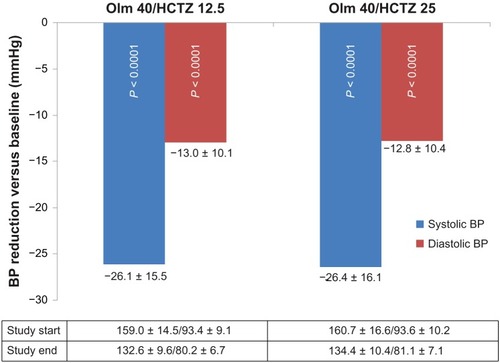
Figure 2 BP target achievement* and BP response** (% of patients).
Abbreviations: BP, blood pressure; LAE, last available examination; Olm, olmesartan; HCTZ, hydrochlorothiazide.
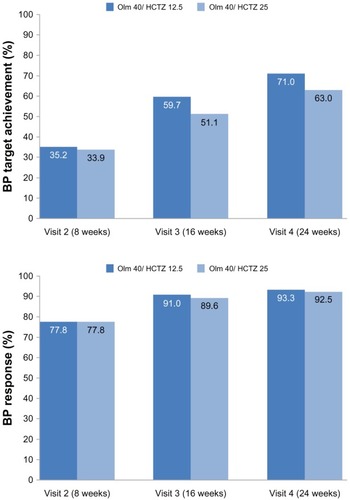
Table 3 Efficacy according to European Society of Hypertension/European Society of Cardiology 2007 blood pressure (BP) categories (n = 3301)
Table 4 Efficacy according to patient subgroup at last available visit (n = 3,301)
Table 5 Efficacy and safety of the fixed-dose combination of olmesartan 40 mg and hydrochlorothiazide 12.5/25 mg in more and less physically active patients (n = 2327)