Figures & data
Table 1 Summary of MI rates in studies of stroke prevention in AF
Table 2 Overview of dabigatran trials included in analysis
Table 3 Summary of studies included in the meta-analyses
Figure 1 (A) Cardiovascular events for dabigatran 150 mg twice daily (n = 10,042) versus warfarin (n = 9,987) in treated patients (randomization to study termination), (B) cardiovascular events for dabigatran 110 mg twice daily (n = 5,983) versus warfarin (n = 5,998) in treated patients (randomization to study termination). (A) The analysis includes RE-LY, RE-MEDY, RE-COVER, and RE-COVER II.Citation1,Citation2,Citation28,Citation30,Citation31 Two studies also compared dabigatran 150 mg twice daily versus warfarin, but no cardiovascular events occurred in these studies and they are not included in this comparison.Citation26,Citation27 Heterogeneity was seen in the following composite endpoints: MI and stroke not leading to vascular death and cardiovascular death, P = 0.07; MI and stroke and vascular death, P = 0.05. (B) This analysis includes RE-LY.Citation1,Citation2 One study also compared dabigatran 110 mg twice daily versus warfarin, but there were no cardiovascular events in that study.Citation27
Abbreviations: bid, twice daily; CI, confidence interval; CV, cardiovascular; MI, myocardial infarction; OR, odds ratio; RE-LY, Randomized Evaluation of Long-term anticoagulation therapY; RE-COVER, A Randomized Trial of Dabigatran Versus Warfarin in the Treatment of Acute Venous Thromboembolism; RE-MODEL, Regulation of Coagulation in Orthopedic surgery to pRevent Deep venous thrombosis and pulmonary embolism; RE-MEDY, A Phase III, Randomised, Multicenter, Double-blind, Parallel-group, Active Controlled Study to Evaluate the Efficacy and Safety of Oral Dabigatran Etexilate (150 mg Bid) Compared to Warfarin (INR 2.0–3.0) for the Secondary Prevention of Venous Thromboembolism.
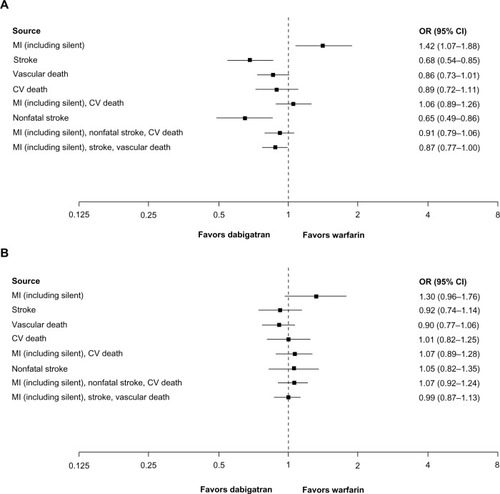
Table 4 Rates of MI and CV events in RE-LY,Citation1,Citation2,Citation12 randomized set
Table 5 Effect of INR control on MI rates in warfarin-treated patients in RE-LY
Table 6 Timing of MI with respect to treatment in RE-LY, randomized set
Table 7 CV events in dabigatran VTE treatment, secondary VTE prophylaxis, and primary VTE prophylaxis in orthopedic surgery trials
Figure 2 CV events for dabigatran 220 mg once daily (n = 3,692) versus enoxaparin (n = 3,719) in treated patients (randomization to study termination). This analysis includes RE-MOBILIZE, RE-MODEL, RE-NOVATE, and RE-NOVATE II.Citation21–Citation23,Citation25
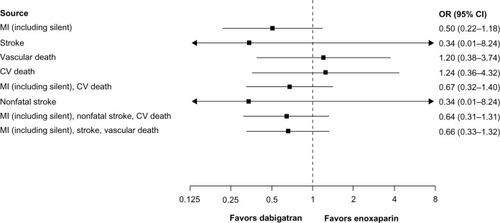
Figure 3 (A) CV events for dabigatran 150 mg twice daily (n = 1,023) versus placebo (n = 1,031) in treated patients (randomization to study termination), (B) CV events for dabigatran 110 mg twice daily (n = 406) versus placebo (n = 371) in treated patients (randomization to study termination). (A) This analysis includes RE-SONATECitation29 and RE-DEEM.Citation32 (B) This analysis includes RE-DEEM.Citation32
Abbreviations: CI, confidence interval; CV, cardiovascular; MI, myocardial infarction; OR, odds ratio; VTE, venous thromboembolism; RE-SONATE, Twice-daily Oral Direct Thrombin Inhibitor Dabigatran Etexilate in the Long Term Prevention of Recurrent Symptomatic VTE; RE-DEEM, RandomizEd Dabigatran Etexilate Dose Finding Study in Patients With Acute Coronary Syndromes Post Index Event With Additional Risk Factors for Cardiovascular Complications Also Receiving Aspirin and Clopidogrel: Multicentre, Prospective, Placebo Controlled, Cohort Dose Escalation Study.
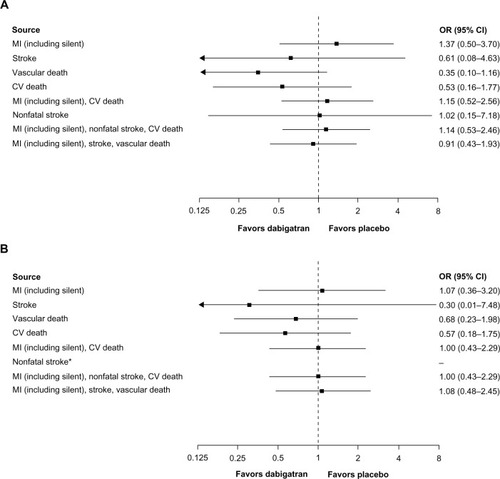
Table S1 Patient characteristics
Table S2 Definition of MI/ACS events
Table S3 Clinical endpoints in RE-DEEMCitation32 patients