Figures & data
Table 1 Pharmacological properties of rivaroxaban, apixaban, and dabigatran etexilate
Table 2 Summary of key findings from the Phase III ROCKET AF trial of rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation
Table 3 Rivaroxaban for stroke prevention in patients with nonvalvular AF – recommendations
Figure 1 Algorithm for use of a reduced dose of rivaroxaban (15 mg once daily instead of 20 mg once daily) in patients with nonvalvular atrial fibrillation, according to the patient characteristics.
Abbreviations: CrCl, creatinine clearance; Hb, hemoglobin.
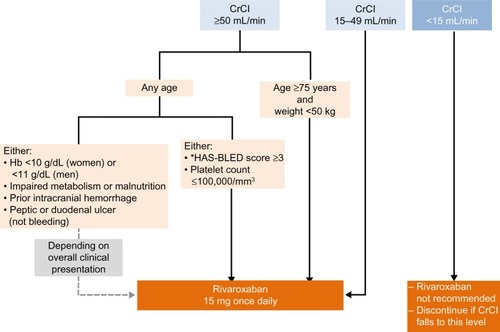
Table 4 Potential interactions between rivaroxaban and the drugs affecting the CYP3A4 and P-gp pathways
Figure 2 (A) Switching from VKAs to rivaroxaban. (B) Switching from rivaroxaban to VKAs.
Abbreviations: CrCl, creatinine clearance; INR, international normalized ratio; od, once daily; VKA, vitamin K antagonist.
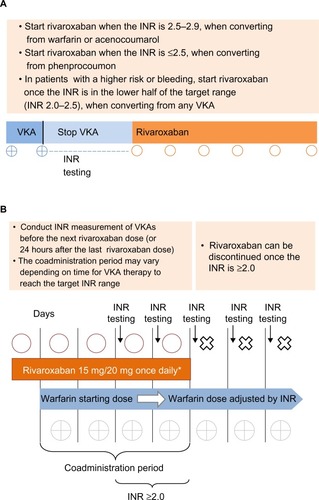
Figure 3 Managing patients requiring an invasive procedure or surgery.
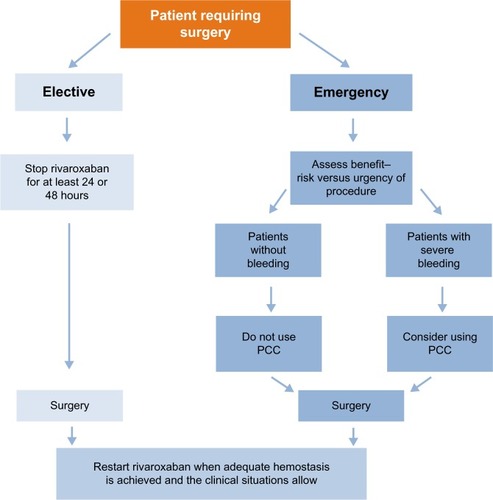
Figure 4 Management of patients with atrial fibrillation experiencing ischemic stroke.
Abbreviation: PT, prothrombin time.
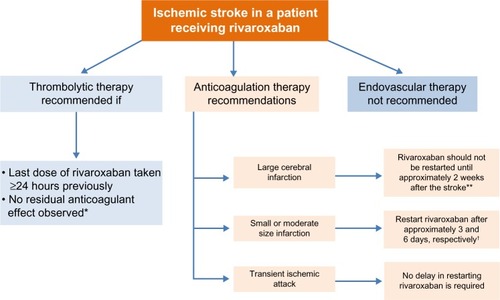
Figure 5 Assessment of bleeding risk in patients receiving rivaroxaban: anticoagulant-related and patient-related risk factors.
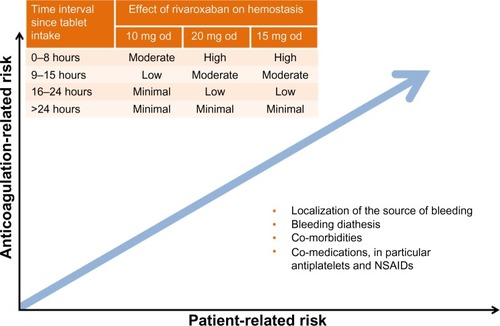
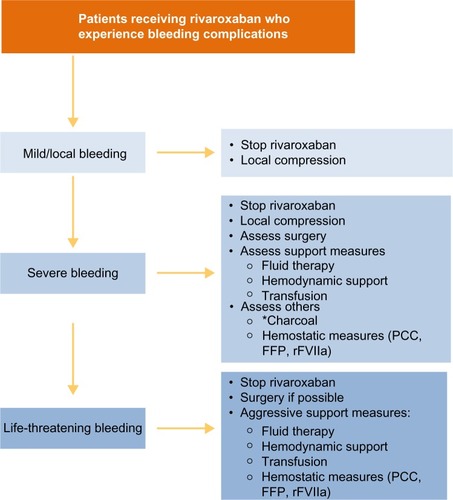
Figure 6 Recommended strategies for managing bleeding events.
Abbreviations: FFP, fresh frozen plasma; PCC, prothrombin complex concentrate; rFVIIa, recombinant factor VIIa.