Figures & data
Figure 1 Patient disposition.
Abbreviations: SBP, systolic blood pressure; V1, baseline; ITT, intention to treat; PP, per protocol.
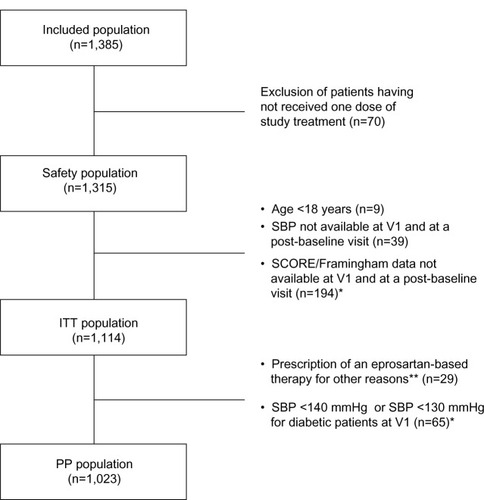
Table 1 Demographic characteristics (ITT population; n=1,114) and Framingham-eligible subset (n=933)
Table 2 Baseline mean blood pressures and hypertension classification (ITT population; n=1,114) and Framingham-eligible subset (n=933)
Figure 2 Baseline Framingham CHD risk status, according to methodology.
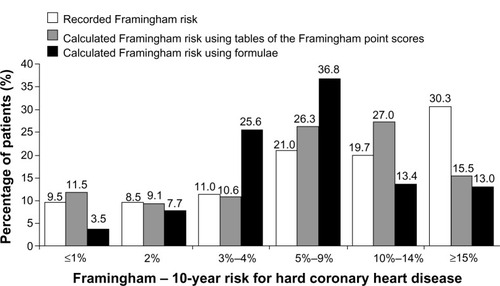
Figure 3 Shifts in Framingham CHD risk distribution during treatment.
Abbreviation: CHD, coronary heart disease.
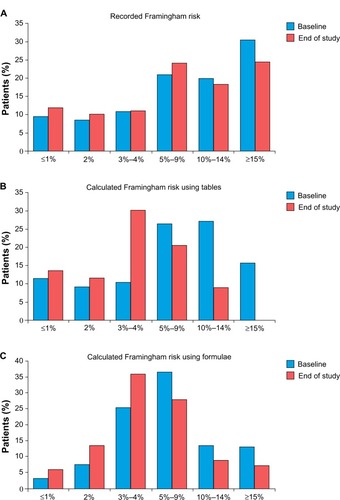
Figure 4 Aggregate shift in Framingham CHD risk distribution during treatment.
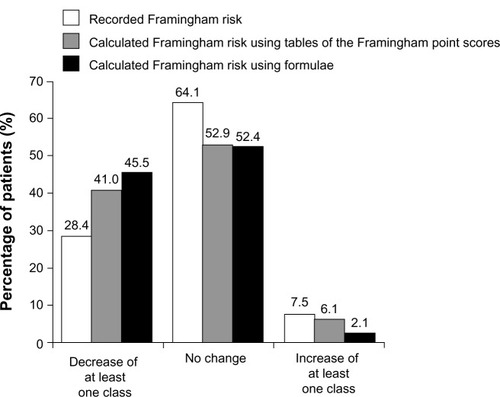
Table 3 Absolute mean change (± SD) of calculated Framingham CHD risk using formula, stratified by age and sex
Table 4 Distribution on change in risk factors (SBP, total and HDL cholesterol, and smoker status) during the survey among patients whose overall CHD risk decreased by at least one class
Table 5 Summary of suspected adverse drug reactions in the Canadian POWER safety population (N=1315)
