Figures & data
Table 1 Clinical studies comprising the XANTUS program
Figure 1 XANTUS study design, an observational, single-arm cohort study. The objective is to collect real-life data on adverse events, bleeding, thromboembolic events, and mortality in patients with nonvalvular AF treated with rivaroxaban. The same basic design will be used for all studies in the XANTUS program.
Abbreviations: AF, atrial fibrillation; CNS, central nervous system; XANTUS, Xarelto® for Prevention of Stroke in Patients with Atrial Fibrillation.
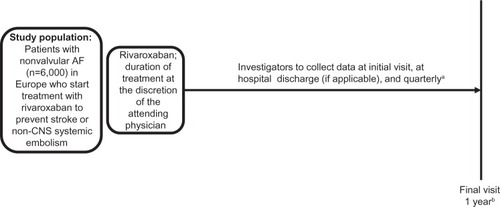
Table S1 Primary efficacy results for ROCKET AF
Table S2 Rates of bleeding outcomes in ROCKET AF
