Figures & data
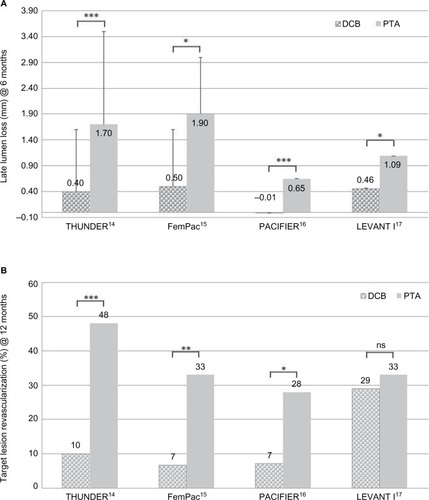
Table 1 Early clinical trials of DCB vs PTA in femoropopliteal lesions
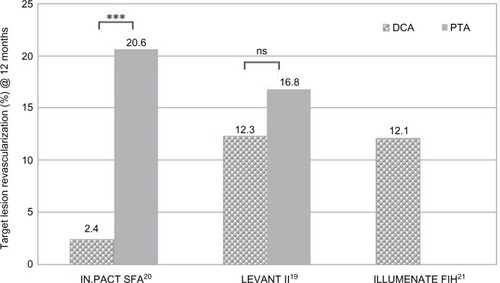
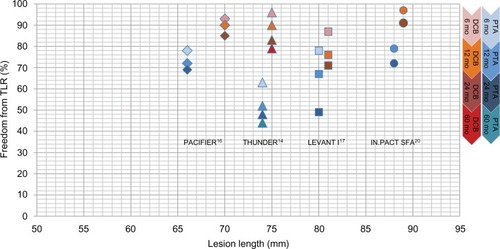
Table 2 Recent clinical trials of DCB vs PTA and registries of DCB use in the SFA
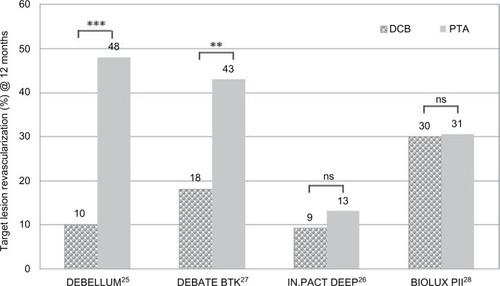
Table 3 Clinical trials of DCB vs PTA in BTK lesions
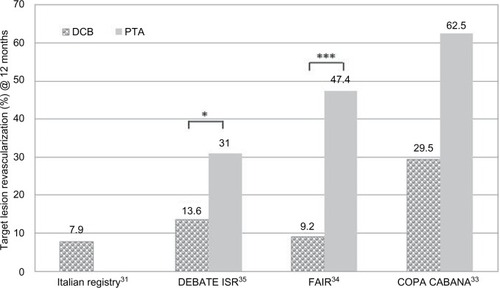
Table 4 Clinical trials of DCB vs PTA in ISR of the SFA
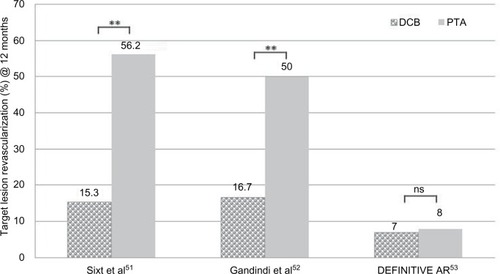
Table 5 Clinical trials combining debulking and DCB in the SFA
TepeGZellerTAlbrechtTLocal delivery of paclitaxel to inhibit restenosis during angioplasty of the legN Engl J Med2008358768969918272892 WerkMLangnerSReinkensmeierBInhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trialCirculation2008118131358136518779447 WerkMAlbrechtTMeyerDRPaclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized PACIFIER trialCirc Cardiovasc Interv20125683184023192918 ScheinertDDudaSZellerTThe LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplastyJACC Cardiovasc Interv201471101924456716 HertenMSchönefeldEStahlhoffSSchwindtATorselloGBDrug-coated balloons in the treatment of femoro- and infra-popliteal lesionsInterv Cardiol201574353370 TepeGLairdJSchneiderPIN.PACT SFA Trial Investigators. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and/or popliteal peripheral artery disease: 12-month results from the IN.PACT SFA Randomized TrialCirculation2015131549550225472980 RosenfieldKJaffMRWhiteCJLEVANT 2 InvestigatorsTrial of a paclitaxel-coated balloon for femoropopliteal artery diseaseN Engl J Med2015373214515326106946 DudaSILLUMENATE FIH: direct DCB cohort – first resultsLINCLeipzig, Germany2015 Available from: http://linc2015.cloudcontrolled.com/media/15th_1_0830_Duda.pdfAccessed February 19, 2015 FanelliFCannavaleABoattaELower limb multilevel treatment with drug-eluting balloons: 6-month results from the DEBELLUM randomized trialJ Endovasc Ther201219557158023046320 LiistroFPortoIAngioliPDrug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemiaCirculation2013128661562123797811 ZellerTBaumgartnerIScheinertDIN.PACT DEEP Trial Investigators. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN.PACT DEEP randomized trialJ Am Coll Cardiol201464151568157625301459 ZellerTBeschornerUPilgerEPaclitaxel-coated balloon in infrapopliteal arteries: 12-month results from the BIOLUX P-II randomized trial (BIOTRONIK’S-first in man study of the Passeo-18 LUX drug releasing PTA balloon catheter vs. the uncoated Passeo-18 PTA balloon catheter in subjects requiring revascularization of infrapopliteal arteries)JACC Cardiovasc Interv20158121614162226493253 StabileEVirgaVSalemmeLDrug-eluting balloon for treatment of superficial femoral artery in-stent restenosisJ Am Coll Cardiol201260181739174223040582 LiistroFAngioliPPortoIPaclitaxel-eluting balloon vs. standard angioplasty to reduce recurrent restenosis in diabetic patients with in-stent restenosis of the superficial femoral and proximal popliteal arteries: the DEBATE-ISR studyJ Endovasc Ther20142111824502477 KrankenbergHTublerTIngwersenMDrug-coated balloon versus standard balloon for superficial femoral artery in-stent restenosis: the randomized femoral artery in-stent restenosis (FAIR) TrialCirculation2015132232230223626446728 TepeGThe Copa Cabana study: DEB vs. POBA in in-stent restenosisLINCLeipzig, Germany2015 Available from: http://linc2015.cloudcontrolled.com/media/15t_1_1620_Tepe.pdfAccessed February 19, 2015 SixtSCarpio CancinoOGTreszlADrug-coated balloon angioplasty after directional atherectomy improves outcome in restenotic femoropopliteal arteriesJ Vasc Surg201358368268623755977 GandiniRDel GiudiceCMerollaSMorosettiDPampanaESimonettiGTreatment of chronic SFA in-stent occlusion with combined laser atherectomy and drug-eluting balloon angioplasty in patients with critical limb ischemia: a single-center, prospective, randomized studyJ Endovasc Ther201320680581424325697 TepeGDCB + atherectomy for complex lesions: DEFINITIVE AR – final dataLINCLeipzig, Germany2015 Available from: http://linc2015.cloudcontrolled.com/media/15t_1_1005_Tepe.pdfAccessed February 19, 2015