Figures & data
Figure 1 The CV continuum. Copyright © 2004, Elsevier. Adapted with permission from Julius S, Kjeldsen SE, Weber M, et al; VALUE trial group. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363(9426):2022–2031.
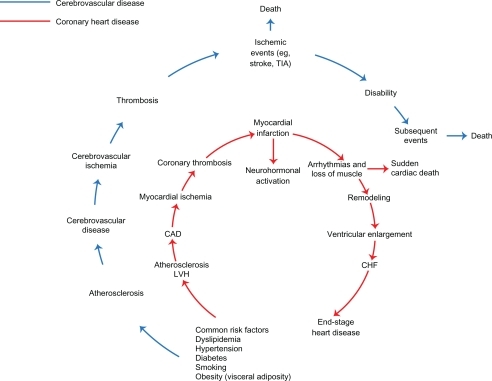
Table 1 Summary of ARB efficacy studies
Figure 2 Kaplan-Meier curves for the primary composite endpoint in the life study: losartan vs atenolol in patients with hypertension and LVH. Copyright © 2002, Elsevier. Adapted with permission from Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003.
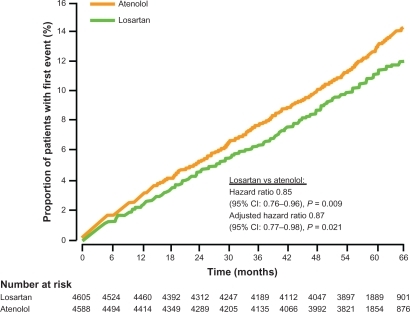
Figure 3 Kaplan-Meier curves of the cumulative frequency of the combined primary endpoint (cv morbidity and mortality) in the jikei heart study: valsartan vs non-ARB treatment. Copyright © 2007, Elsevier. Adapted with permission from Mochizuki S, Dahlöf B, Shimizu M, et al. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2007;369(9571):1431–1439.
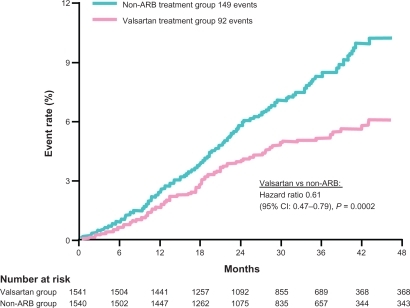
Figure 4 Kaplan-Meier curves for the primary outcome (death from CV causes, MI, Stroke, or hospitalization for HF) in ONTARGET. Copyright © 2008, Massachusetts Medical Society. Adapted with permission from ONTARGET Investigators, Yusuf S, Teo KK, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559.
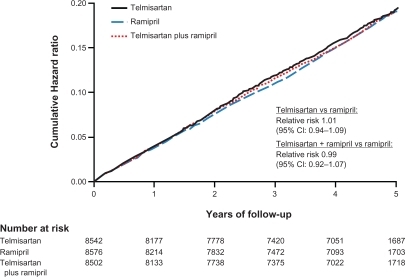
Figure 5 Kaplan-Meier estimates of the rate of death from any cause in the VALIANT Study: valsartan, captopril or their combination in Post-MI patients. Copyright © 2003, Massachusetts Medical Society. Adapted with permission from Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893–1906.
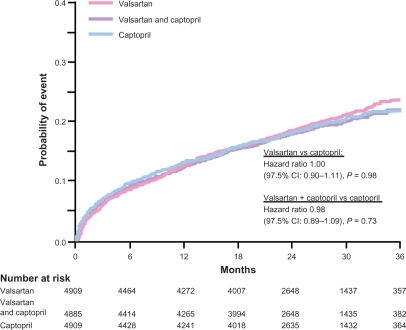
Figure 6 Kaplan-Meier Cumulative Event Curves for the Combined Primary Endpoint (CV Morality + Hospitalization for Heart Failure): Candesartan vs Placebo in ACE-inhibitor-intolerant Patients – CHARM-alternative. Copyright © 2003, Elsevier. Adapted with permission from Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362(9386):772–776.
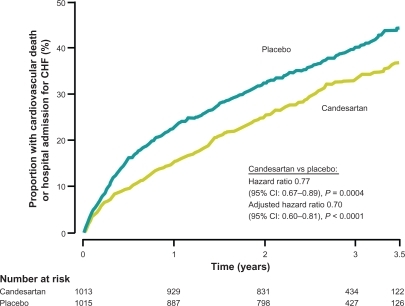
Table 2 Ongoing ARB trials with primary CV endpoints across the CV continuum
Table 3 Functional capacity at the various treatment steps (mean ± SD)
Table 4 Maximal exercise blood pressure and heart rate and oxygen reserve and LVM at the various treatment